Figure 4.
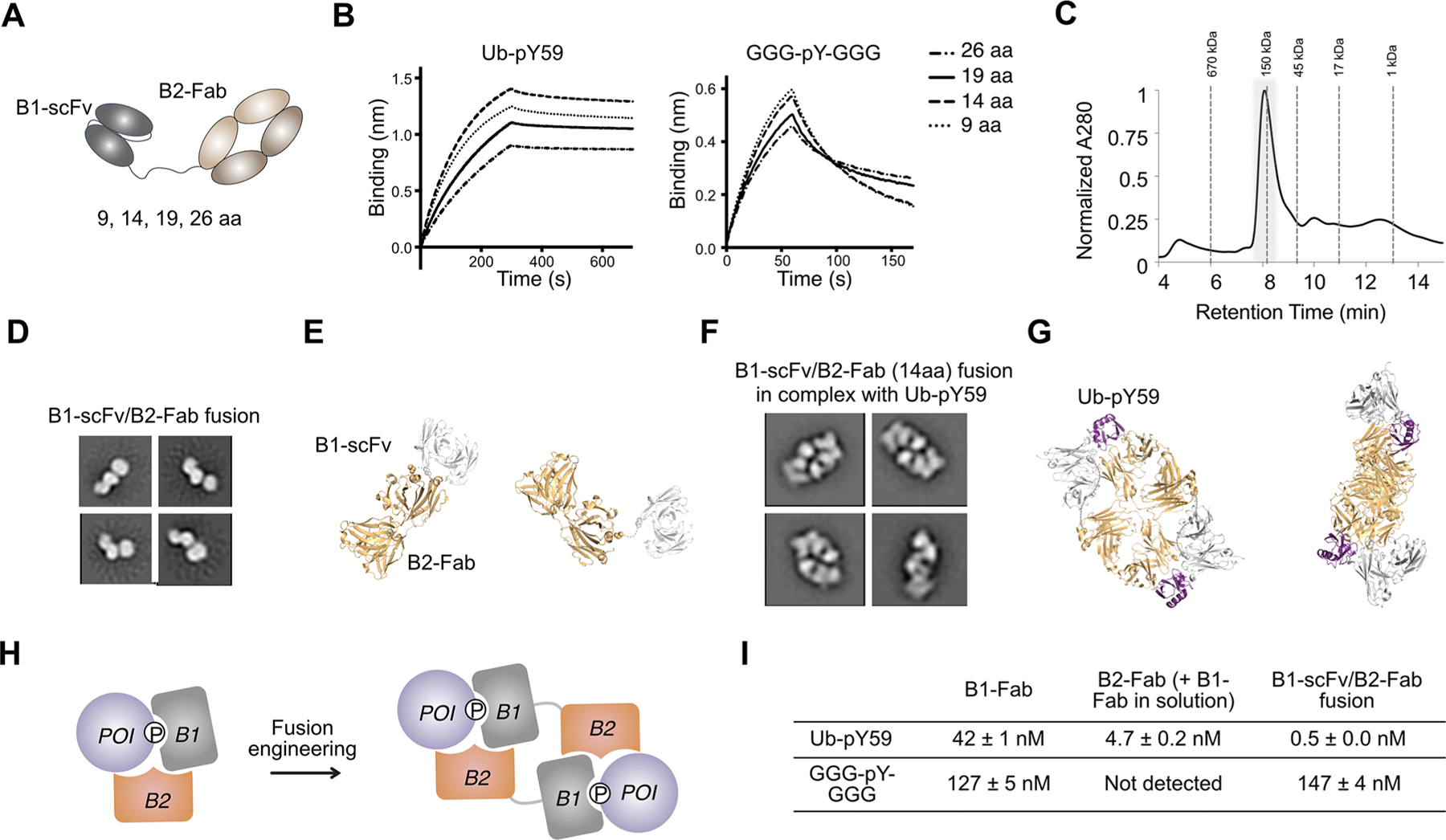
B1-scFv/B2-Fab fusion engineering. (A) Cartoon illustration of the B1-scFv/B2-Fab fusion. Various linker lengths (9, 14, 19, and 26 residues) were tested in the fusion protein between B1 and B2. (B) BLI experiments show that B1-scFv/B2-Fab with a 14-aa linker has the biggest difference in binding affinity for Ub-pY59 and GGG-pY-GGG. (C) The SEC analysis of the complex of B1-scFv/B2-Fab and Ub-pY59 shows that the complex has a molecular weight ∼150 kDa. The peak (highlighted in gray background) is collected for SDS-PAGE and NS-EM analysis. (D) Representative 2D class averages of NS-EM data for the B1-scFv/B2-Fab fusion alone. (E) Structural models of the B1-scFv/B2-Fab fusion. Light orange, Fab, PDB 1N8Z; gray, scFv, PDB 6DF1. (F) Representative 2D class averages of NS-EM data for B1-scFv/B2-Fab in complex with Ub-pY59. (G) Structure models of a 2:2 dimer of B1-scFv/B2-Fab and Ub-pY59. B1-scFv and B2-Fab in one polypeptide chain interact with two different Ub-pY59 molecules. Light orange, Fab, PDB 1N8Z; gray, scFv, PDB 6DF1; purple, Ub, PDB 5XK5. (H) Cartoon illustration of the formation of a 2:2 dimer of B1-scFv/B2-Fab and Ub-pY59. (I) Summary of KD values for B1-Fab, B2-Fab, and B1-scFv/B2-Fab to Ub-pY59 and the GGG-pY-GGG peptide.
