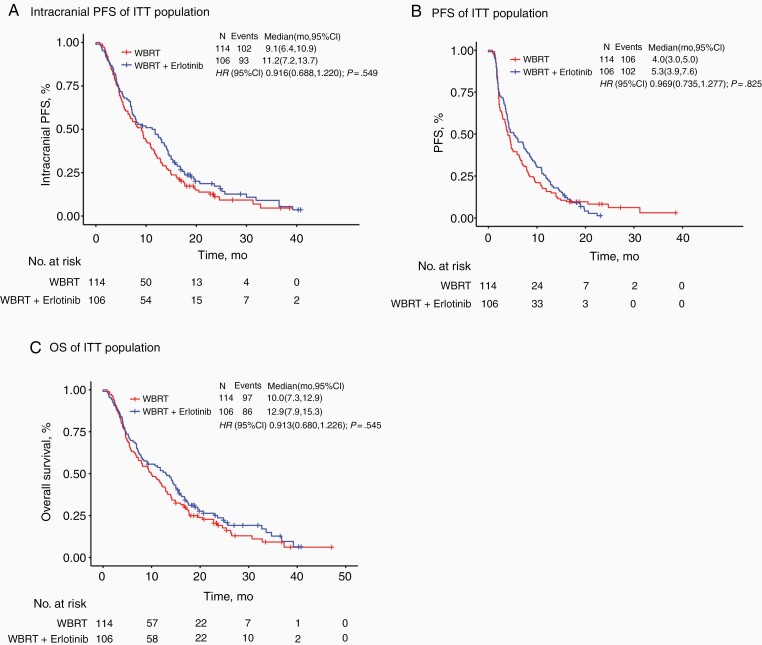
Fig. 2.
Kaplan-Meier survival curves for (A) intracranial progression-free survival (iPFS), (B) progression-free survival (PFS), and (C) overall survival (OS) in the intent-to-treat (ITT) study population. (A) Median iPFS in the ITT population was 11.2 months (95% CI: 7.2-13.7) in the WBRT with concurrent erlotinib arm vs 9.1 months (95% CI: 6.4-10.9) for WBRT-alone. (B) Median PFS of concurrent erlotinib compared with WBRT-alone was 5.3 months vs 4.0 months, respectively. There was no statistically significant difference in PFS between the two treatment arms (HR, 0.969; 95% CI: 0.735-1.277; P = .825). (C) Median OS was 12.9 vs 10.0 months for WBRT with concurrent erlotinib and WBRT-alone, respectively. There was no statistically significant difference in OS between the two treatment arms (HR, 0.913; 95% CI: 0.680-1.226; P = .545). CI, confidence interval; WBRT, whole-brain radiotherapy; HR, hazard ratio.