Abstract
Carbon-fiber microelectrodes (CFMEs) have been used for several years for the detection of neurotransmitters such as dopamine. Dopamine is a fundamentally important neurotransmitter and is also metabolized at a subsecond timescale. Recently, several metabolites of dopamine have been shown to be physiologically important such as 3-methoxytyramine (3-MT), 3,4-dihydroxyphenylacetic acid (DOPAC), and homovanillic acid (HVA). Many of these neurotransmitter metabolites are currently only detected with microdialysis coupled with liquid chromatography with relatively low temporal and spatial resolution. Current electrochemical methods such as the dopamine waveform (scanning from −0.4 to 1.3 V at 400 V/sec) are utilized to electrostatically repel anions such as DOPAC and promote dopamine adsorption to the surface of the electrode. Moreover, polymer coatings such as Nafion have been shown to electrostatically repel anions such as 5-hydroxyindoleacetic acid (5-HIAA). In this study, we develop novel polymer and waveform modifications for enhanced DOPAC detection. Applying the DOPAC waveform (scanning from 0 to 1.3 V at 400 V/sec) enhances DOPAC detection significantly because it does not include the negative holding potential of the dopamine waveform. Moreover, positively charged cationic polymers such as polyethyleneimine (PEI) allow for the preconcentration of DOPAC to the surface of the carbon fiber through an electrostatic attraction. The limit of detection for DOPAC for PEI coated CFMEs with the DOPAC waveform applied is 58.2 ± 2 nM as opposed to 291 ± 10 nM for unmodified electrodes applying the dopamine waveform (n = 4). This work offers promise for the development of novel electrode materials and waveforms for the specific detection of several important biomolecules such as dopamine metabolite neurotransmitters.
Graphical Abstract
Introduction
Carbon-fiber microelectrodes (CFMEs) have been utilized for the past forty years as the standard for neurotransmitter detection such as dopamine.1 Dopamine is a crucially important neurotransmitter for understanding sex2, learning, motivation, movement, Parkinson’s disease3, drug abuse4–6, and other disorders.7 The physical and chemical properties of CFMEs make them ideal electrode materials for dopamine detection. First and foremost, carbon fibers are made from loosely ordered sheets of graphene that are pyrolyzed from polyacrylonitrile (PAN) at high temperatures. Other carbon fibers are pitch-based and have varying response to cations and anions based on their related conductivities.8 The thermal and electrochemical etching of carbon fibers breaks carbon-carbon bonds and increases surface roughness, hence increasing the electroactive surface area.9, 10 Moreover, electrochemical etching also functionalizes the end groups of carbon fibers (primarily basal plane carbon) with negatively charged carboxylate (carboxylic acid), hydroxyl, and oxide groups at the end of edge plane carbon.11 The negative charge of the functionalized carbon fibers are conducive for the detection of dopamine. At a physiological pH of 7.4, the amine of dopamine is protonated, thus making it a positively charged cationic molecule.12, 13 The cationic dopamine adsorbs to the surface of the negatively charged carbon fiber through an electrostatic interaction, which allows for adsorption and preconcentration onto the surface of the electrode and enhances the sensitivity of neurotransmitter detection.
Recently, the physiological importance of dopamine metabolites has also been realized. Dopamine is metabolized on a subsecond timescale. Pre-synaptically, dopamine is metabolized to 3,4-dihydroxyphenylacetic acid (DOPAC) via monoamine oxidase (MAO), while it is metabolized post-synaptically to 3-methoxytyramine (3-MT) by catechol-o-methyltransferase (COMT).14 Furthermore, both DOPAC and 3-MT are been further metabolized to homovanillic acid (HVA) by the enzymes, COMT and MAO, respectively.15 The depletion of norepinephrine (another metabolite of dopamine) was postulated to cause Parkinson’s Disease until Hornykewicz and colleagues observed significantly lower levels of dopamine in the basal ganglia and degeneration of nigrostriatal dopamine-containing neurons in post-mortem brains of patients16. Although 3-MT was once thought to be physiologically inactive,17 multiple studies have shown that it is an important neuromodulator in the brain.14, 17 3-MT has also been found to be an important neuromodulator and agonist of the human trace amine associated receptor (TAAR1).15 DOPAC detection has also been vital for understanding and studying Parkinsonism,18, 19 foot-shock,20 conditioned stress,21 and other drug related effects.22, 23 MAO24 and COMT25 inhibitors such as entacapone are potent therapeutics utilized for Parkinson’s disease, which highly regulate DOPAC levels by altering dopamine metabolism. The sensitivity for dopamine detection at CFMEs is over ten times greater with respect to DOPAC even though DOPAC is found in higher concentrations in certain brain regions due to the electrostatic repulsion of the negatively charged DOPAC at the surface of the negatively charged oxide-functionalized CFME.26, 27
Analytical method development has frequently been utilized to enhance the detection of neurotransmitters and metabolites. Altering the waveform applied and the chemical and physical properties of the electrode materials can allow for fine tuning the electrochemical sensitivity for enhanced neurotransmitter selectivity and detection. Novel waveform modifications have been developed for the electrochemical detection of neurotransmitters such as dopamine,9, 10 serotonin,28 adenosine,29, 30 histamine,31 tyramine,32 octopamine,33 hydrogen peroxide,34, 35 and neuropeptides,36, 37 among others. Moreover, polymer and other electrode coatings such as Nafion,38, 39 poly(3,4-ethylenedioxythiophene) PEDOT-Nafion,40, 41 overoxidized-polypyrrole,42 vertically aligned carbon nanotube forests,43 functionalized carbon nanotubes,44 and others have been used to enhance neurotransmitter detection by increasing conductivity and reducing surface fouling.45
In this study, we utilize a combination of waveform modifications and polymer coatings to test for the enhancement of DOPAC selectivity and discrimination from dopamine when detected with CFMEs using FSCV. Polymer coatings such as polyethyleneimine (PEI) and Nafion were used to functionalize the surface of the CFMEs to discriminate dopamine and DOPAC. PEI coatings on the surface of CFMEs applied a more positive charge to the surface of the electrode due the protonation of the nitrogen functionalized groups, which electrostatically attract the negatively charged anionic DOPAC. Conversely, electrodeposition of Nafion onto the surface of the CFME coated the electrode with a thin negatively charged polymer that electrostatically attracts dopamine, but repels DOPAC. Moreover, we have developed the novel “DOPAC waveform” that is similar to the traditional “dopamine waveform10” except that it has a holding potential of 0 V instead of −0.4 V. This prevents that electrostatic repulsion of DOPAC from the surface of the electrode at the negative holding potential. This work will provide a novel enhancement for DOPAC selectivity, detection at subsecond timescale, and a possibly future greater understanding of this important metabolite for in vivo measurements.
Results and Discussion
Surface Characterization of Polymer Coated CFMEs
As with dopamine, DOPAC oxidation is a two-electron process where the catechol is oxidized to a quinone (Figure 1). The process is quasi-reversible where not all of the DOPAC that is oxidized is reduced back down to DOPAC on the reverse scan. The only structural difference between dopamine and DOPAC is the presence of a carboxyl group instead of an amine. This functional group is of vital importance in understanding the electron transfer kinetics and adsorption of dopamine to the surface of carbon fibers or polymer coated fiber materials. At a physiological pH of 7.4, the amine of dopamine is protonated to give it an overall positive charge. At the same pH, the carboxyl group of DOPAC is deprotonated giving it an overall negative charge. In order to develop waveforms and electrode materials that are conducive to DOPAC rather than dopamine, the overall negative charge of the electrode must be reduced, and a positive charge must be applied instead.
Figure 1:
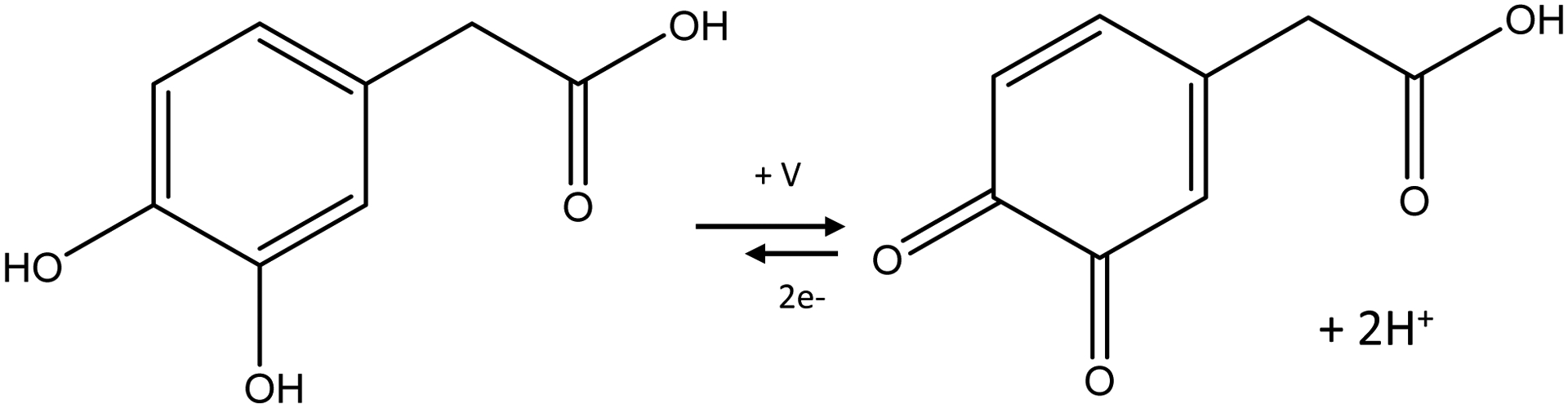
Schematic of DOPAC Oxidation. Similar to dopamine, DOPAC is also a catechol that undergoes reversible oxidation and reduction in a two-electron transfer. Unlike dopamine, DOPAC contains a carboxyl group instead of an amine and is negatively charged at a physiological pH.
As shown in the Scanning electron microscopy (SEM) images, the bare carbon fiber microelectrodes have deep grooves and ridges as they are thermally spun from polyacrylonitrile (PAN) and pyrolyzed at temperatures above 1,000 K.1, 8 These bare unmodified CFMEs are approximately 7 microns in diameter and were utilized for control testing comparisons with polymer modified microelectrodes (Figure 2A). Furthermore, CFMEs were electrodeposited with either Nafion (Figure 2B) or polyethyleneimine (Figure 2C). CFMEs were dipped into a 5% weight perfluorinated Nafion resin in aliphatic alcohols and electrodeposited upon applying a + 1 V potential. As shown in Figure 2B, a thin and uniform layer of Nafion was electrodeposited throughout the entire surface of the carbon fiber. Nafion is a commonly utilized polymer (ionomer) that allows for the movement of cations, but not anions. Nafion coatings create a thin, uniform negatively charged electrode surface that will electrostatically attract the positively charge cationic dopamine, while electrostatically repelling anions such as DOPAC, uric acid (UA), or ascorbic acid (AA).
Figure 2:
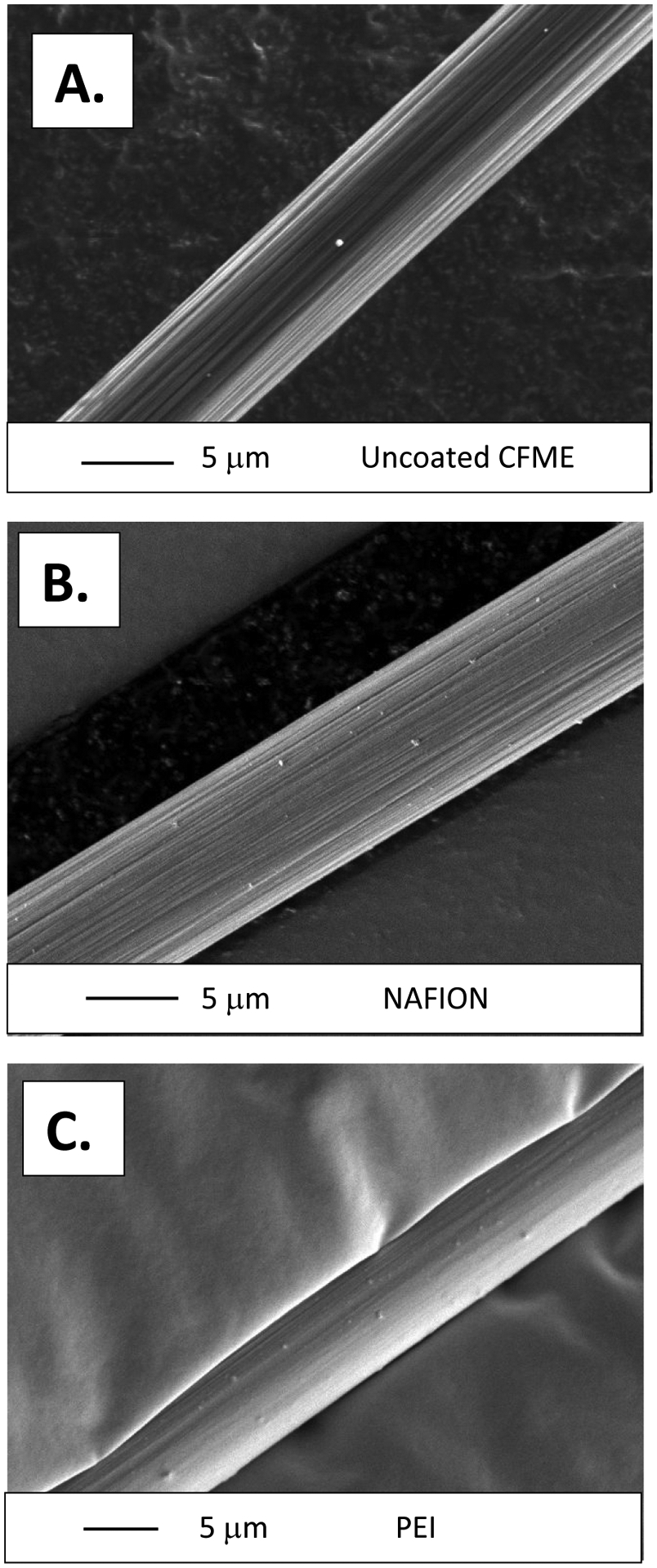
SEM Images of A). A bare uncoated carbon fiber approximately 7 microns in diameter. B). A carbon fiber electrodeposited in a Nafion solution for approximately 5 minutes. A thin layer of polymer evenly coats the surface of the electrode. C). Polyethyleneimine (PEI) Coated Carbon fibers. The disappearance of the ridges in Figures 2B and 2C indicate that the carbon fibers have been coated in a thin layer of polymer. Small dots on the surface of the fibers are remnants of gold sputtering that were utilized to increase the conductivity, and hence resolution, of the images.
Moreover, polyethyleneimine (PEI, 5,000 Mn, 20% in methanol, Sigma-Aldrich, Milwaukee) was also electrodeposited onto the surface of CFMEs. The amine group of PEI is protonated and hence positively charged at a physiological pH of 7.4 of phosphate buffered saline (PBS). Therefore, we hypothesize that PEI coatings onto the surface of CFMEs will electrostatically attract anions such as DOPAC allowing for adsorption, while lowering sensitivity for other cations through electrostatic repulsion. PEI was electrodeposited onto the surface of CFMEs using the triangle waveform by cycling from 1.5 V to −0.8 V to 1.5 V at a 100 mV/sec scan rate for approximately 300 s. The electrodes were then dried for approximately 1 hour in the oven at 80°C. The PEI polymer aggregated onto the surface of CFMEs to form a complete and thorough coating on the surface (Fig. 2C). Gold sputtering was utilized to enhance the conductivity, and hence resolution, of the polymer coated fibers. PEI modified microelectrodes have been shown to enhance neurotransmitter detection when used to form carbon nanotube (CNT fiber) microelectrodes.46 The amine group of the PEI is hypothesized to physisorb to the surface of the graphitic carbon, thus inducing an intermolecular charge transfer and making a more conductive substrate that can be used as an electrode material for neurotransmitter detection with FSCV.47 Furthermore, EDS spectra were also collected from the images of the polymer-coated CFMEs. The presence of C, N, and O confirmed the identity of the deposited PEI polymer (Supplementary Information Fig. 1A), while presence of C and F confirmed the deposition of Nafion onto the surface of the carbon fiber (Supplementary Information Fig. 1B).
Comparison of polymer coatings for Dopamine and DOPAC detection.
After constructing and modifying CFMEs with Nafion and PEI, we compared the sensitivity of these modified electrodes for DOPAC detection. As expected, bare CFMEs had higher sensitivity towards DOPAC in comparison to Nafion coated CFMEs. The oxide groups of CFMEs are negatively charged, thus allowing for dopamine preconcentration and adsorption to the surface, enhancing detection, and allowing for lower limits of detection and higher sensitivities. However, this does not hold true for DOPAC, which is electrostatically repelled by the negatively charged Nafion. On the other hand, PEI coatings also allowed for higher sensitivity measurements of DOPAC vs. bare and Nafion coated microelectrodes.
We then determined the effect of the electrodeposition of Nafion and PEI polymers on the surface of CFMEs on the detection of dopamine, DOPAC, ascorbic acid, and uric acid using FSCV. In Figure 3, we show example cyclic voltammograms of 1 μM Dopamine (DA), 5 μM DOPAC, 200 μM ascorbic acid (AA), and 20 μM uric acid (UA) on bare and polymer coated microelectrodes. These are concentrations of neurotransmitters within the broad physiological range as shown in the literature.46, 48 The application of Nafion coatings had no significant effect on the sensitivity for dopamine detection (Figure 3A). On the other hand, the negatively charged Nafion significantly reduced the sensitivity for DOPAC detection (Figure 3B, p = .0415, n = 4, t-test), ascorbic acid (Figure 3C, p = .0025, n = 4, t-test), and (uric acid, p = .0012, n = 4, t-test) as shown with example cyclic voltammograms of uncoated and Nafion coated CFMEs to the left and normalized (dividing all peak oxidative currents by the largest current and multiplying by 100) averages to the right. As expected, the negatively charged Nafion electrostatically repels the negatively charged anionic molecules from the surface of the carbon fiber, thus significantly reducing sensitivity in comparison to bare uncoated CFMEs
Figure 3. Effect of Polymer Coatings on Dopamine, DOPAC, Ascorbic Acid, and Uric Acid Detection.
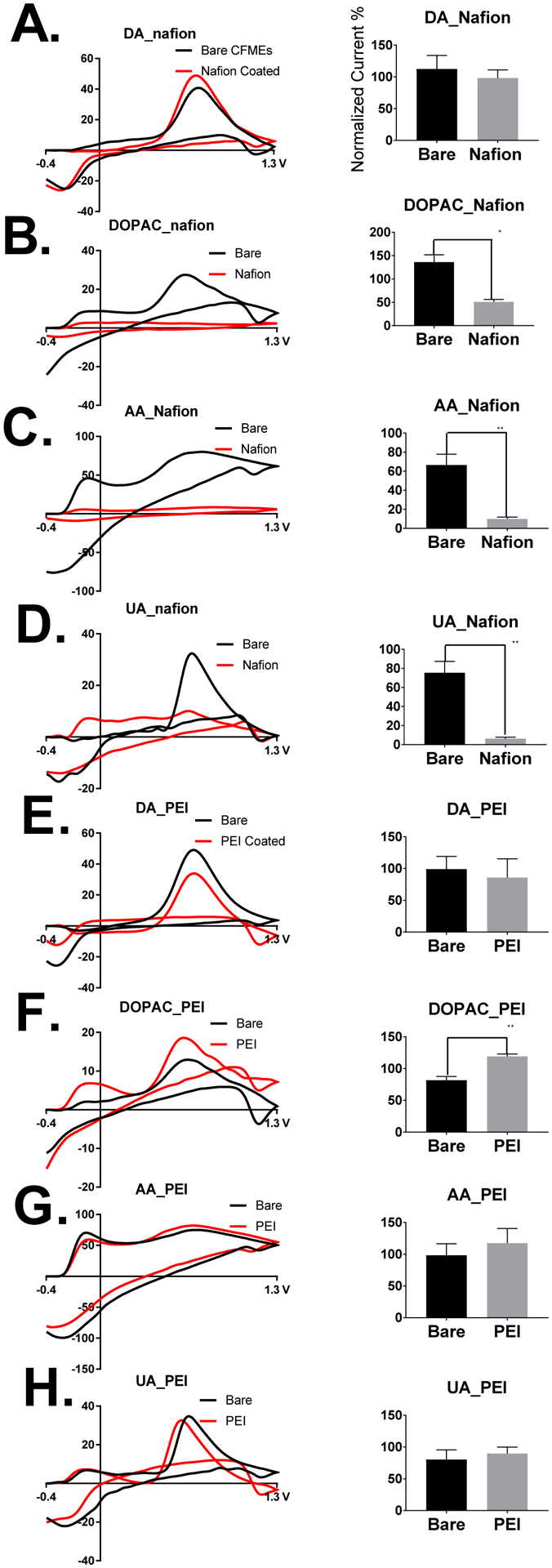
Example cyclic voltammograms of 1 μM Dopamine (DA), 5 μM DOPAC, 200 μM ascorbic acid (AA), and 20 μM uric acid (UA) on bare and polymer coated microelectrodes. The effect of Nafion coatings in comparison to uncoated electrodes on the sensitivity for 3A. Dopamine detection, 3B. DOPAC detection (t-test, p = .0415), 3C. Ascorbic Acid (t-test, p = .0025), and 3D. Uric Acid (t-test, p = .0012) is shown above with example cyclic voltammograms of uncoated and Nafion coated CFMEs to the left and normalized averages to the right. Negatively charged coatings electrostatically repel anions (DOPAC, ascorbic acid, and uric acid) from the surface of the electrode and hence decrease sensitivity. The effect of PEI coatings on sensitivity for 3E. dopamine detection, 3F. DOPAC detection (t-test, p = .0057), 3G. ascorbic acid, and 3H. uric acid are shown above with example cyclic voltammograms of uncoated and PEI coated CFMEs to the left and normalized averages to the right (n = 4). PEI coatings on the surface of CFMEs enhance the sensitivity of DOPAC detection, but not the other analytes.
The effect of PEI coatings on the sensitivity for the same four aforementioned molecules were then compared to bare uncoated CFMEs as shown in the example cyclic voltammograms (left) and bar charts (right) shown in Figures 3 E–H. The electrodeposition of PEI polymer onto the surface of the electrode had no effect on the sensitivity of detection for dopamine (Figure 3E) ascorbic acid (Figure 3G), or uric acid (Figure 3H). On the other hand, the electrodeposition of PEI onto the surface of CFMEs significantly enhanced DOPAC detection (p = .0057, n = 4, t-test). The explanation for these phenomena has yet to be determined. We hypothesize that the positively charged PEI polymer, protonated at a physiological pH, electrostatically attracts the negatively charged DOPAC (deprotonated and negatively charged at a physiological pH of 7.4). The same observation does not hold true for uric acid for ascorbic acid, most likely because the catechol Hs of DOPAC induce hydrogen bonding interactions and the phenol of DOPAC undergoes π–π stacking interactions with graphitic edge plane carbon, which is not present in either uric acid or ascorbic acid. Furthermore, we also hypothesize that PEI coatings on carbon fibers are not thick enough to electrostatically repel dopamine from the surface of the carbon fiber to decrease sensitivity because the carbon fiber (functionalized with negatively charged oxide groups, Supplementary Information Figure 1A) and the dopamine waveform (including the negative holding potential) are optimal for dopamine detection, which overcome any influence of the PEI coating to possibly repel dopamine. Previous studies have shown that wetspun PEI-carbon nanotube fiber microelectrodes enhance the detection and co-detection of dopamine and serotonin most likely due to an intermolecular charge transfer from the imine of the PEI to the carbon fiber, thus making a more conductive electrode substrate.46, 47
In comparison to dopamine oxidation, the peak shape for DOPAC CVs are much broader due to slower electron transfer kinetics from electrostatic repulsion. Electrodeposition with PEI increases peak oxidative current possibly due to functionalizing the surface of the electrode with a slight positive charge, thus reducing the electrostatic repulsion. PEI contains positively charged imine groups that are protonated in the physiological buffer, phosphate buffered saline (PBS). Therefore, it electrostatically attracts the negatively charged DOPAC allowing for increased adsorption to the surface of the electrode, and thus providing for a markedly higher sensitivity with respect to uncoated CFMEs.
Effect of Polymer Coatings on Time Response
It is expected that the deposition of polymer onto the surface of electrode will markedly increase the response time of the electrode for the respective analyte. This phenomenon occurs because the polymer creates an extra layer through which the analyte must diffuse in order to adsorb onto the surface of the carbon-fiber microelectrode. This observation has been noted in studies concerning the electrodeposition of Nafion onto the surface of CFMEs for enhanced serotonin detection,38 enzyme immobilization for glucose biosensing,49 glucose oxidase,50 and chitosan coatings51 for CFMEs. To examine this, we performed several flow injection analysis experiments to determine the effect of polymer coatings on time response. As expected, the bare unmodified CFME had the fastest time response to 5 μM DOPAC and yielded a relatively square current vs. time (I vs. T) trace (Supplementary Information Fig. 2A). The PEI coated CFME had a slightly slower and more “curved” I vs. T trace (Supplementary Information Fig. 2B). The current vs. time trace for the Nafion coated CFMEs (Supplementary Information 2C) were more “pointed” and had the greatest deviation from the square current vs. time trace of uncoated CFMEs. The slower time response for polymer coated electrodes was expected, though the polymer coated electrodes should still be able to detect fast changes of neurochemicals in vivo.
It has been previously shown that pH shifts have been known to occur when detecting acidic compounds such as DOPAC at relatively high concentrations.26 To check for pH shifts, we performed flow injection analysis experiments where we diluted .1 M (.1 N) perchloric acid in PBS buffer (1.25 mM phosphate) without the presence of the neurotransmitter stock solution similar to a flow injection analysis experiment for dopamine. The cyclic voltammogram produced yielded a strong peak at −0.1 V, comparable to pH shifts for DOPAC found in the literature as shown in the Supplementary Information Figure 3.26 After increasing the concentration of buffer to 12.5 mM phosphate, the peak disappeared, thus allowing us to hypothesize that a more concentrated buffer prevents the occurrence of pH shifts during experimentation.
Flow cell characterization of polymer coated CFMEs
After noticing the increase in sensitivity for detection of PEI coatings for DOPAC, we wanted to determine whether this coating affected adsorption control of either dopamine (1 μM) or DOPAC (5 μM) to the surface of the carbon fiber-microelectrode. To determine this, we performed several experiments including stability, scan rate, and concentration testing. As shown in Figure 4, PEI coatings did not have any effect on the adsorption control of dopamine or DOPAC to the surface of CFMEs, thus illustrating their utility as coatings for enhanced neurochemical detection. PEI coated CFMEs displayed a stability towards dopamine and DOPAC response in the flow cell for a period of at least four hours, which is the typical duration of an in vivo experiment with CFMEs (Figure 4A). Moreover, we also show that peak oxidative current of DOPAC cyclic voltammograms are also linear with respect to scan rate for the detection of dopamine and DOPAC at CFMEs. We also altered the scan rates from 50 V/sec to 1,000 V/sec and observed a linear relationship with respect to scan rate and peak oxidative current for both dopamine (1 μM) and DOPAC (5 μM) that denoted adsorption control to the surface of the electrode (Figure 4B). For more diffusion controlled analytes, peak oxidative current would be proportional to the square root of scan rate. Furthermore, we also observed a linear relationship for concentration (up to 10 μM) and peak oxidative current (Figures 4C and 4D). After 10 μM, both DOPAC and dopamine become saturated at the surface of the electrode where all adsorption sites have been occupied, which explains the asymptotic curve at higher concentrations. This explains the asymptotic curve for higher concentrations of these neurotransmitters.
Figure 4. Adsorption Control Testing of the Polymer Coated CFMEs.
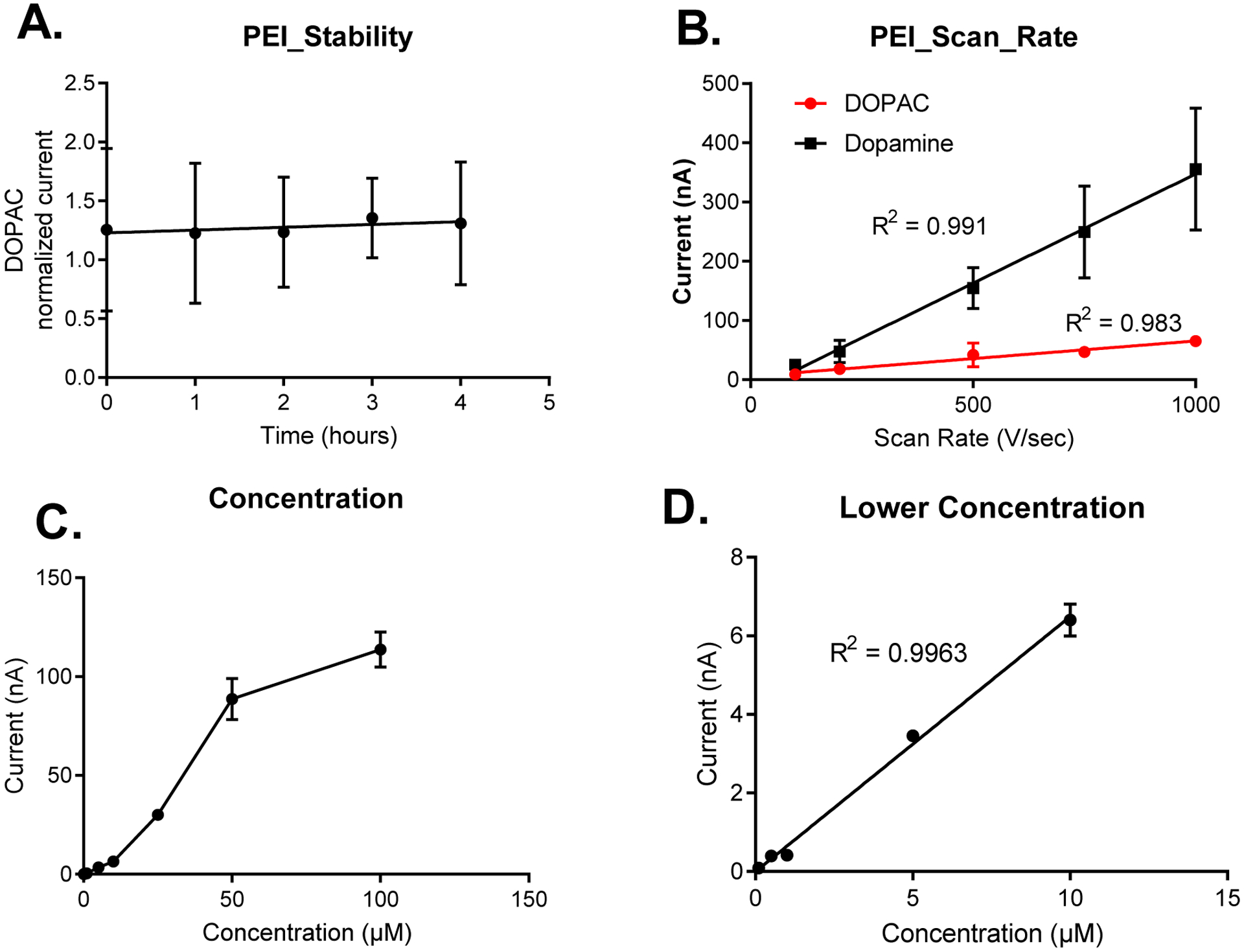
4A. The PEI coated electrodes display a stability towards DOPAC detection (peak oxidative current) for at least four hours. 4B. Scan rate. The peak oxidative current for dopamine (1 μM) and DOPAC (5 μM) cyclic voltammograms are linear with respect to scan rate ( 50 V/sec – 1,000 V/sec), thus denoting adsorption control to the surface of the polymer coated CFMEs. Figure 4C. The peak oxidative currents for the cyclic voltammograms for DOPAC are linear with respect to concentration up from 100 nM to 10 μM. At higher concentrations, DOPAC is saturated at the surface of the electrode, which blocks sites for further adsorption, hence the asymptotic curve. Figure 4D, shows that concentration is linear with respect peak oxidative current at lower concentrations (at 10 μM or less), R2 = 0.9963 (n = 3).
DOPAC Waveform Testing
The “dopamine” waveform applied a potential that scans from −0.4 V to 1.3 V, at 400 V/sec has long been seen as the ideal waveform for dopamine detection with fast scan cyclic voltammetry.10 Scanning to 1.3 V renews the surface of the electrode through electrochemical etching, which prevents analyte saturation, surface fouling, functionalizes the electrode surface with negatively charged oxide groups (oxide, hydroxy, carbonyl, carboxyl, ketones, etc.),11 and breaks carbon-carbon bonds to increase the surface roughness and, hence, the electroactive surface area to make the electrode more sensitive for dopamine detection.10 Setting the holding potential to −0.4 V makes the electrode surface more negative, which allows for the adsorption of cationic catecholamines such as dopamine.12, 13 However, upon applying the dopamine waveform, the CFMEs become less sensitive for anionic analytes such as DOPAC and ascorbic acid because the negatively charged electrode electrostatically repels the like-charged DOPAC (Figure 3).
In order to increase the sensitivity for DOPAC measurements with CFMEs and FSCV, we increased the holding potential (lower limit) of the waveform from −0.4 V to −0.1 V, 0 V, and 0.1 V, respectively as shown in the modified triangle waveform in Figure 5A. We purposefully chose a positive, neutral, and negative potential limit to test our electrostatics hypothesis on the sensitivity of DOPAC detection for CFMEs using FSCV. The positive (0.1 V) and neutral (0 V) potential lower limit waveforms have average peak oxidative current markedly higher than that of the negative holding potential, −0.1 V (Figure 5B, One-way ANOVA, Tukey’s multiple comparisons test, (−0.1 V vs. 0 V, p = .0161) and (−0.1 V vs. 0.1 V, p = .0174), n=3 for both. Example cyclic voltammograms for 10 μM DOPAC using the varied potential limits (holding potential) are shown in Figure 5C (scanning from −.1 V to 1.3 V), Figure 5D (scanning from 0 V to 1.3 V), and Figure 5E (scanning from .1 V to 1.3 V). As expected, the positive and neutral waveforms produced significantly higher peak oxidative currents than the waveform with the negative holding potential, which most likely electrostatically repels the negatively charged DOPAC molecule from the surface of the electrode. Therefore, we chose the DOPAC waveform to be from 0 V to 1.3 V because 0 V is the highest potential lower limit that does not reduce the sensitivity for DOPAC detection. One consequence of increasing the potential lower limit though was the loss of the reduction peak of DOPAC since the DOPAC waveform does not contain a negative holding potential of −0.4 V unlike the dopamine waveform, which could potentially hinder selectivity with respect to multiplexing with other analytes. However, several analytes such as adenosine,29, 52 adenosine triphosphate (ATP),30 and hydrogen peroxide34, 35, 53–55 are selectively detected without the presence of a reduction peak.
Figure 5.
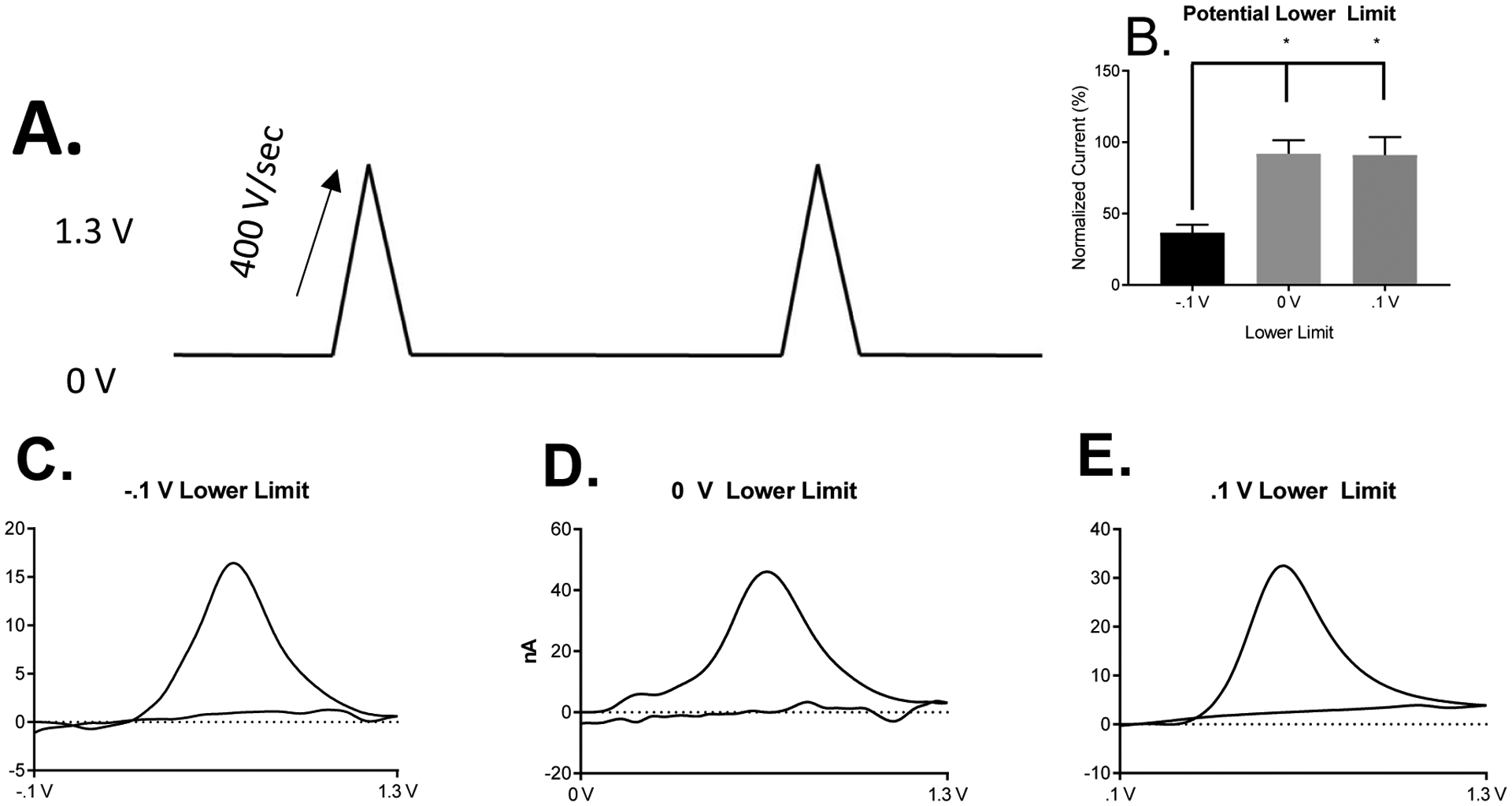
DOPAC Voltammetry at DOPAC Waveform on Uncoated CFMEs. A. The proposed DOPAC waveform is similar to the “Dopamine” waveform except that the holding potential is 0 V and not −0.4 V. The waveform applied scans from 0 V to 1.3 V at 400 V/sec. B. Comparison of 10 μM DOPAC percent normalized (dividing all peak oxidative currents by the largest current and multiplying by 100) detection using the positive, neutral, and negative potential lower limits. The positive and neutral lower limit waveforms have average peak oxidative current markedly higher than the positive waveform, One-way ANOVA, Tukey’s multiple comparisons test. (−.1 vs. 0 V, p = .0161), (−.1 V vs. .1 V, p = .0174), n=3 for both. Example Cyclic Voltammogram of 10 μM DOPAC using the varied lower potential limits (holding potential). C. Scanning from −.1 V to 1.3 V. D. Scanning from 0 V to 1.3 V. E. Scanning from .1 V to 1.3 V
Characterization of DOPAC using the DOPAC Waveform.
Using the DOPAC waveform, we also tested for adsorption control of DOPAC to the surface of the CFME. Since DOPAC is no longer electrostatically repelled by the negatively charged electrode, it is, thus, more likely to adsorb to the surface of a neutral or positively charged electrode surface. As shown in Figure 6, the response to DOPAC and dopamine using the DOPAC waveform was stable over a period of four hours, which is the typical length of an in vivo experiment (Figure 6A). Also, when using the DOPAC waveform, concentration was proportional to peak oxidative current (from 500 nM – 10 μM). Upon increasing the concentration of dopamine and DOPAC from 10 μM to 100 μM, the analytes also became saturated at the surface of the electrode because all the sites for adsorption at the surface of the electrode have become occupied. Furthermore, peak oxidative currents of dopamine and DOPAC were also found to be linear with respect to scan rate upon varying the scan rate from 50 V/sec to 1,000 V/sec. Therefore, this illustrates that both DOPAC and dopamine were found to be adsorption controlled at the surface of the CFME even in the absence of a negative holding potential. If DOPAC were diffusion controlled at the surface of the electrode, then peak oxidative current would be proportional to the square root of scan rate, which was not observed in this case. We hypothesize that adsorption occurs at the surface of the electrode possibly through a π–π stacking interaction mechanism and hydrogen bonding (from the catechol Hs) of the phenyl group of DOPAC and dopamine to the oxide groups that are present on the surface of the carbon fiber microelectrode when utilizing the DOPAC waveform.
Figure 6. Adsorption Control Testing of the DOPAC waveform applied to Uncoated CFMEs.
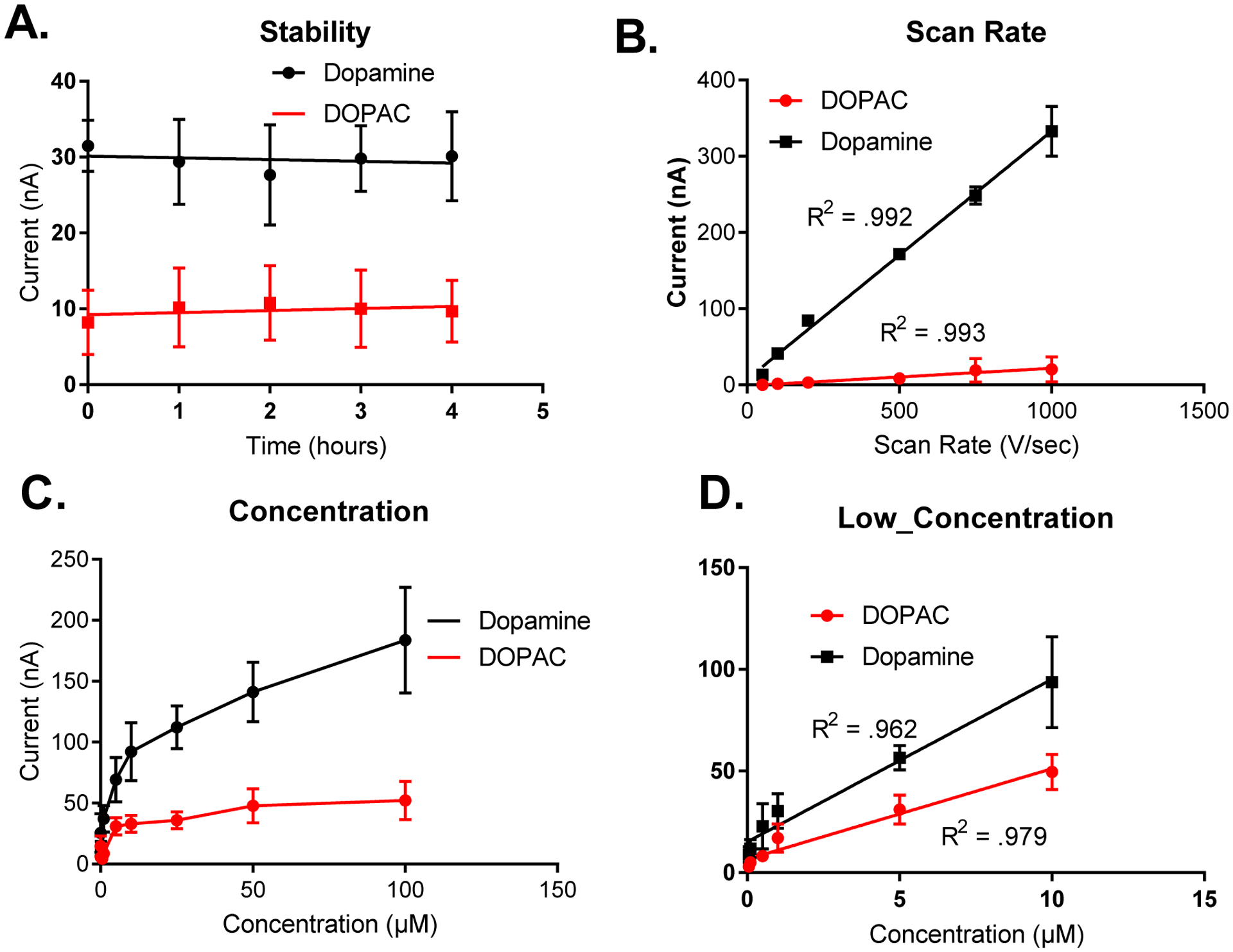
6A. Applying to the DOPAC waveform electrodes display a stability towards dopamine (1 μM) and DOPAC (5 μM) detection (peak oxidative current) for at least four hours. 6B. Scan rate. The peak oxidative current for dopamine (1 μM) and DOPAC (5 μM) cyclic voltammograms are linear with respect to scan rate ( 50 V/sec – 1,000 V/sec), thus denoting adsorption control to the surface of CFMEs when the DOPAC waveform is applied. Figure 6C. The peak oxidative currents for the cyclic voltammograms for dopamine and DOPAC are linear with respect to scan rate up from 500 nM to 10 μM. At higher concentrations, dopamine is saturated at the surface of the electrode, which blocks sites for further adsorption, hence the asymptotic curve. Figure 6D shows that both peak oxidative currents for both DOPAC and dopamine are linear at lower concentrations, up to 10 μM where the analytes are not yet saturated at the surface of the electrode.
Co-Detection of Dopamine and DOPAC
Lastly, we also tested the combination of the DOPAC waveform and polymer coatings to the surface of CFMEs for both dopamine and DOPAC sensitivity. As expected, PEI coated electrodes had significantly higher sensitivities for DOPAC detection with respect to bare CFMEs utilizing the dopamine waveform. The limit of detection for DOPAC with the PEI coated CFMEs and the DOPAC waveform applied is 58.2 ± 2 nM as opposed to 291 ± 10 nM for unmodified electrodes applying the dopamine waveform (n = 4). Again, the positive charge applied to the surface of the electrode is hypothesized to induce an electrostatic attraction with the negatively charged anionic DOPAC. Also, the DOPAC waveform does not contain the negative holding potential (potential lower limit) of the dopamine waveform, which would electrostatically repel DOPAC from the surface of the electrode. As shown in Figure 7A, a mixture containing 1 μM DOPAC and 1 μM dopamine was measured using FSCV and DOPAC and dopamine were differentiated and co-detected when placed in the flow cell together and analyzed with PEI-CFMEs with dopamine waveform applied. The dimple in the oxidation peak for the cyclic voltammogram allows dopamine to be differentiated from DOPAC. We hypothesize that the application of the PEI coatings must have altered the electron transfer kinetics of dopamine and DOPAC to the surface of the CFME to have caused this peak shift. The electron transfer kinetics of DOPAC to the surface of the electrode are slower on CFMEs due to the presence of negatively charged oxide groups at the surface which electrostatically repel anions form the surface. The right shift of the reduction peak relative to dopamine in the cyclic voltammograms denotes the presence of DOPAC. We have ascertained that dopamine is the peak on the left as increasing concentrations of dopamine used in the mixture increase the left peak (2 μM dopamine: 1 μM DOPAC, Figure 7B), while increasing concentrations of DOPAC (1 μM dopamine: 2 μM DOPAC) increase the right peak (Figure 7C). Despite the presence of the PEI polymer, we hypothesize that dopamine still has faster electron transfer kinetics at the surface of the carbon fiber due to the presence of negatively charged oxide groups, which still slightly repel DOPAC adsorption from the surface as shown in the EDS measurements of the PEI coated carbon fiber (Supplementary Information, Figure 1A). The converse is also true when testing with lower concentrations of both DOPAC and dopamine reduce the peak oxidation currents of the right and left peaks, respectively. Furthermore, PEI-CNT fiber microelectrodes have been used to differentiate and co-detect mixtures of serotonin and dopamine.46 We hypothesize that the altered electrode surface of the PEI polymer allows for differential adsorption of both dopamine and DOPAC to the surface of the CFME.
Figure 7. Co-detection of Dopamine and DOPAC using PEI Electrodes and the Dopamine Waveform.
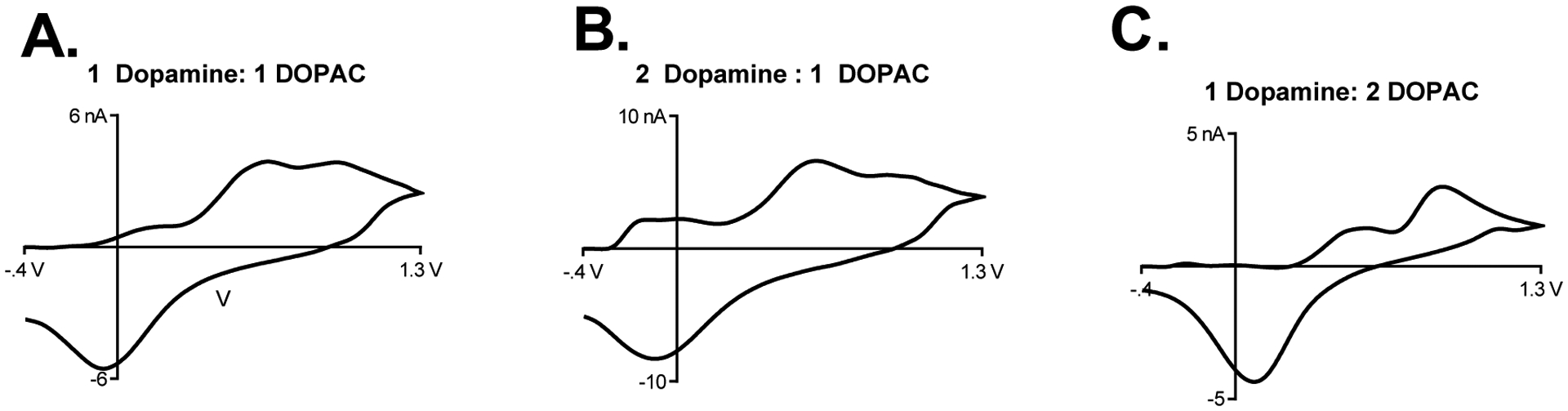
Various concentrations of dopamine and DOPAC were prepared in a solution of buffer and injected into the flow cell and were detected with PEI coated CFMEs utilizing the dopamine waveform. A. The cyclic voltammogram of a solution containing 1 μM dopamine and 1 μM DOPAC. B. The cyclic voltammogram of a solution containing 2 μM dopamine and 1 μM DOPAC. C. The cyclic voltammogram of a solution containing 1 μM dopamine and 2 μM DOPAC The peak oxidative currents of DOPAC (right) and dopamine (left) can be clearly distinguished from one another using PEI modified CFMEs.
Methods and Materials
Dopamine, DOPAC, ascorbic acid, and uric acid were obtained from Sigma-Aldrich (St. Louis, MO). A 10 mM stock solution was prepared in 0.1 M perchloric acid and diluted to 1.0 – 100 μM daily with phosphate-buffered saline (PBS) (131.5 mM NaCl, 3.25 mM KCl, 1.2 mM CaCl2, 12.5 mM NaH2PO4 1.2 mM MgCl2, and 2.0 mM Na2SO4 with the pH adjusted to 7.4). All aqueous solutions were made with deionized water (Millipore, Billerica, MA). Epon 828 Epoxy was obtained from Miller-Stephenson (Morton Grove, IL) and Diethylenetriamine hardener was obtained from Fisher Scientific (Waltham, MA).
Instrumentation
Fast Scan Cyclic Voltammetry (FSCV) was performed with the WaveNeuro FSCV system with a 5 MΩ headstage (Pine Instruments, Durham, NC, USA). Data was collected using HDCV software (University of North Carolina Chapel Hill, Mark Wightman) and a computer interface board (National Instruments PC1e-6363, Austin, TX, USA).
A triangle waveform was applied to the electrode from a holding potential of −0.4 V or 0 V to 1.3 V and back at a scan rate of 400 V/s and a frequency of 10 Hz unless otherwise noted. A silver-silver chloride wire was used as the reference electrode. Samples were tested in a flow injection analysis system (In-Vitro/FSCV Microelectrode Flow Cell with xyz micromanipulator Translational Stage, Pine Instruments, Durham, NC). Buffer and samples were pumped through the flow cell at 2 mL/min unless otherwise noted using the NE-300 Just Infusion™ Syringe Pump (New Era Pump Systems, Farmingdale, NY).
For the traditional waveform, the electrode was scanned from −0.4 to 1.3 V vs. silver-silver chloride (Ag/AgCl, .197) reference electrode and back at a scan rate of 400 V/s and a wave application frequency of 10 Hz. The waveform for enhanced DOPAC detection was scanned from 0 V to 1.3 V at 400 V/sec and a wave application frequency of 10 Hz. Both the scan rates (100 – 1,000 V/sec) and concentrations (100 nM – 100 μM) were varied from lower to higher values only. The electrodes were allowed to equilibrate for approximately 10 min to allow the CFME to equilibrate at the waveform applied and prevent electrode drift between each run. A 5 kHz low pass filter was used for experiments with scan rates greater than 400 V/s. All data was background subtracted to remove any non-Faradaic currents by averaging 10 CVs. Electrodes were tested at a flow rate of 2 mL/min using the aforementioned syringe pump.
Electrode Construction
Carbon fibers (.007 mm, Goodfellow, Huntingdon, England) were aspirated into cylindrical glass capillaries (1.2 mm by 0.68 mm, A-M Systems, Inc., Carlsborg, WA) using a vacuum pump (DOA-P704-AA, GAST, Benton Harbor, MI) to form carbon-fiber microelectrodes. The capillary was pulled to form two electrodes on a vertical pipette puller (Narishige, model PC-100 and PE-22, Tokyo, Japan), and the fiber cut to lengths of approximately 100–150 microns. Glass insulated electrodes were epoxied with Epon 828 epoxy (Miller-Stephenson, Morton Grove, IL) and diethylenetriamine (Sigma Aldrich, Milwaukee, WI). Protruding carbon-fiber microelectrode tips were dipped in the epoxy hardener mixture (0.8% by mass resin) for approximately 15 seconds and then rinsed in acetone to wash away any excess residual acetone.56 The electrodes were cured in the oven for 3 h at 165°C.
Electrodeposition of polymers onto CFMEs
The electrodeposition of Nafion® perfluorinated resin solution, 5 wt % in lower aliphatic alcohols and water onto the surface of carbon-fiber microelectrodes (CFMEs) was performed as previously described.38 The carbon-fiber microelectrode was immersed in a Nafion perfluorinated resin solution (5% weight in lower aliphatic alcohols and water, Sigma-Aldrich, Milwaukee, WI), and a potential of 1 volt was applied vs. Ag/AgCl reference electrode for approximately 60 s. The CFMEs were then dried in the over 1 h at 80°C.
CFMEs were also modified in polyethyleneimine (PEI) polymer. Linear PEI polymer (Mn 5,000, Sigma Aldrich, Milwaukee, WI) was dissolved in methanol to make a 20% mass solution. The CFME was lowered into the PEI solution. A triangle waveform scanning from +1.5 V to −0.8V and back to +1.5 V was applied a scan rate of 100 mV/s. Electrodeposition occurred over five minutes. The CFMEs were cycled with the waveform applied in a 25 mL beaker containing the PEI polymer dissolved in methanol with respect to the Ag/AgCl reference electrode (0.197 V).
Scanning Electron Microscopy
Scanning electron microscopy images (SEM) images were obtained with a JEOL JSM-IT100 (JEOL, Tokyo, Japan). Bare or polymer modified carbon fiber microelectrodes were sputter-coated with gold in Denton Desk II sputter coater at 100 millitorr and 45 milliamps current. They were then placed onto conductive tape, which was then inserted into the stage. The working distance was set to 10 mm and slightly adjusted to obtain optimal resolution and magnification, while the accelerating voltage was 5 kV. Furthermore, the same JEOL software was also used to perform Energy-dispersive x-ray spectroscopy (EDS/EDX) measurements for chemical identification of polymers on the surface of the carbon-fiber microelectrode. The collection time was approximately three minutes.
Data Analysis
All data analysis was performed in Graph Pad Prism 7. The limit of detection was extrapolated to a concentration where S/N =3. Statistical analysis was performed with either a student’s t-test or one-way ANOVA. Statistical significance was set to p < 0.5. All error bars are standard error of the mean (SEM) unless otherwise noted.
Conclusions
As we continue to gain a further understanding of the physiological importance of the neurotransmitter metabolites of dopamine, there exists a greater need to detect these metabolites selectively with a high temporal resolution approaching the rate that they are enzymatically metabolized. This study has depicted the usage of polymer and waveform modifications as methods for enhancing the detection of neurotransmitter metabolites using fast scan cyclic voltammetry. PEI coatings on the surface of carbon electrodes were shown to enhance DOPAC detection through electrostatic interactions of the amine to the negatively charged carboxyl groups. Furthermore, utilizing the DOPAC waveform has also significantly enhanced DOPAC detection by not applying the negative holding potential, which will electrostatically repel anionic metabolites such as DOPAC. The combined usage of novel waveforms and polymer modifications have significantly increase high sensitivity and temporal resolution measurements of DOPAC at lower limits of detection and allows for the differentiation between DOPAC and dopamine when tested in varying ratios in the flow cell. Future work includes measuring multiple neurotransmitter metabolites such as 3-methoxytyramine, homovanillic acid, norepinephrine, normetanephrine, and others in addition to performing in vivo measurements.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the Faculty Research Support Grant at American University, NASA-CAS Fellowship, and NSF-MRI #1625977. We also acknowledge Andrea Brothers for her assistance with gold sputtering and SEM/EDS measurements.
Footnotes
Conflicts of Interest
There are no conflicts to declare.
References
- 1.Huffman ML and Venton BJ, Analyst, 2009, 134, 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morissette M and Di Paolo T, Neuroendocrinology, 1993, 58, 16–22. [DOI] [PubMed] [Google Scholar]
- 3.Kim J-H, Auerbach JM, Rodríguez-Gómez JA, Velasco I, Gavin D, Lumelsky N, Lee S-H, Nguyen J, Sánchez-Pernaute R and Bankiewicz K, Nature, 2002, 418, 50. [DOI] [PubMed] [Google Scholar]
- 4.Zestos AG, Carpenter CA, Kim Y, Low MJ, Kennedy RT and Gnegy ME, ACS Chemical Neuroscience, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carpenter C, Zestos AG, Altshuler R, Sorenson RJ, Guptaroy B, Showalter HD, Kennedy RT, Jutkiewicz E and Gnegy ME, Neuropsychopharmacology, 2017, 42, 1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zestos AG, Mikelman SR, Kennedy RT and Gnegy ME, ACS chemical neuroscience, 2016, 7, 757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zarate CA, Singh JB, Carlson PJ, Quiroz J, Jolkovsky L, Luckenbaugh DA and Manji HK, Bipolar disorders, 2007, 9, 561–570. [DOI] [PubMed] [Google Scholar]
- 8.Huffman ML and Venton BJ, Electroanalysis: An International Journal Devoted to Fundamental and Practical Aspects of Electroanalysis, 2008, 20, 2422–2428. [Google Scholar]
- 9.Keithley RB, Takmakov P, Bucher ES, Belle AM, Owesson-White CA, Park J and Wightman RM, Analytical chemistry, 2011, 83, 3563–3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takmakov P, Zachek MK, Keithley RB, Walsh PL, Donley C, McCarty GS and Wightman RM, Analytical chemistry, 2010, 82, 2020–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts JG, Moody BP, McCarty GS and Sombers LA, Langmuir, 2010, 26, 9116–9122. [DOI] [PubMed] [Google Scholar]
- 12.Bath BD, Martin HB, Wightman RM and Anderson MR, Langmuir, 2001, 17, 7032–7039. [Google Scholar]
- 13.Bath BD, Michael DJ, Trafton BJ, Joseph JD, Runnels PL and Wightman RM, Analytical chemistry, 2000, 72, 5994–6002. [DOI] [PubMed] [Google Scholar]
- 14.Wood PL and Altar CA, Pharmacological Reviews, 1988, 40, 163–187. [PubMed] [Google Scholar]
- 15.Sotnikova TD, Beaulieu J-M, Espinoza S, Masri B, Zhang X, Salahpour A, Barak LS, Caron MG and Gainetdinov RR, PLoS One, 2010, 5, e13452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K and Seitelberger F, Journal of the neurological sciences, 1973, 20, 415–455. [DOI] [PubMed] [Google Scholar]
- 17.Karoum F, Chrapusta SJ and Egan MF, Journal of neurochemistry, 1994, 63, 972–979. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein D, Holmes C, Kopin I and Sharabi Y, Neurology, 2016, 86, I1. 006. [Google Scholar]
- 19.Goldstein D, Holmes C, Stefani A and Sharabi Y, Autonomic Neuroscience: Basic and Clinical, 2015, 192, 129. [Google Scholar]
- 20.Deutch AY, Tam S-Y and Roth RH, Brain research, 1985, 333, 143–146. [DOI] [PubMed] [Google Scholar]
- 21.Fadda F, Argiolas A, Melis MR, Tissari A, Onali P and Gessa G, Life sciences, 1978, 23, 2219–2224. [DOI] [PubMed] [Google Scholar]
- 22.Zetterström T, Sharp T, Collin A and Ungerstedt U, European journal of pharmacology, 1988, 148, 327–334. [DOI] [PubMed] [Google Scholar]
- 23.Fornstedt B, Rosengren E and Carlsson A, Neuropharmacology, 1986, 25, 451–454. [DOI] [PubMed] [Google Scholar]
- 24.Liebowitz MR, Hollander E, Schneier F, Campeas R, Welkowitz L, Hatterer J and Fallon B, Acta Psychiatrica Scandinavica, 1990, 82, 29–34. [DOI] [PubMed] [Google Scholar]
- 25.Nissinen E, Nissinen H, Larjonmaa H, Väänänen A, Helkamaa T, Reenilä I and Rauhala P, Journal of neural transmission, 2005, 112, 1213–1221. [DOI] [PubMed] [Google Scholar]
- 26.Takmakov P, Zachek MK, Keithley RB, Bucher ES, McCarty GS and Wightman RM, Analytical chemistry, 2010, 82, 9892–9900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heien ML, Johnson MA and Wightman RM, Analytical chemistry, 2004, 76, 5697–5704. [DOI] [PubMed] [Google Scholar]
- 28.Jackson BP and Wightman RM, Brain research, 1995, 674, 163–166. [DOI] [PubMed] [Google Scholar]
- 29.Pajski ML and Venton BJ, ACS chemical neuroscience, 2010, 1, 775–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ross AE and Venton BJ, Analytical chemistry, 2014, 86, 7486–7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Denno ME, Privman E, Borman RP, Wolin DC and Venton BJ, ACS chemical neuroscience, 2016, 7, 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fang H, Pajski ML, Ross AE and Venton BJ, Analytical Methods, 2013, 5, 2704–2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooper SE and Venton BJ, Analytical and bioanalytical chemistry, 2009, 394, 329–336. [DOI] [PubMed] [Google Scholar]
- 34.Roberts JG, Hamilton KL and Sombers LA, Analyst, 2011, 136, 3550–3556. [DOI] [PubMed] [Google Scholar]
- 35.Sanford AL, Morton SW, Whitehouse KL, Oara HM, Lugo-Morales LZ, Roberts JG and Sombers LA, Analytical chemistry, 2010, 82, 5205–5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karra S, Griffith WP, Kennedy RT and Gorski W, Analyst, 2016, 141, 2405–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt AC, Dunaway LE, Roberts JG, McCarty GS and Sombers LA, Analytical chemistry, 2014, 86, 7806–7812. [DOI] [PubMed] [Google Scholar]
- 38.Hashemi P, Dankoski EC, Petrovic J, Keithley RB and Wightman R, Analytical chemistry, 2009, 81, 9462–9471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hashemi P, Walsh PL, Guillot TS, Gras-Najjar J, Takmakov P, Crews FT and Wightman RM, ACS chemical neuroscience, 2011, 2, 658–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Demuru S and Deligianni H, Journal of The Electrochemical Society, 2017, 164, G129–G138. [Google Scholar]
- 41.Vreeland RF, Atcherley CW, Russell WS, Xie JY, Lu D, Laude ND, Porreca F and Heien ML, Analytical chemistry, 2015, 87, 2600–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peairs MJ, Ross AE and Venton BJ, Analytical Methods, 2011, 3, 2379–2386. [Google Scholar]
- 43.Xiao N and Venton BJ, Analytical chemistry, 2012, 84, 7816–7822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jacobs CB, Vickrey TL and Venton BJ, Analyst, 2011, 136, 3557–3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang L, Liu D, Huang J and You T, Sensors and Actuators B: Chemical, 2014, 193, 166–172. [Google Scholar]
- 46.Zestos AG, Jacobs CB, Trikantzopoulos E, Ross AE and Venton BJ, Analytical chemistry, 2014, 86, 8568–8575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muñoz E, Suh DS, Collins S, Selvidge M, Dalton AB, Kim BG, Razal JM, Ussery G, Rinzler AG and Martínez MT, Advanced Materials, 2005, 17, 1064–1067. [Google Scholar]
- 48.Yang C, Jacobs CB, Nguyen MD, Ganesana M, Zestos AG, Ivanov IN, Puretzky AA, Rouleau CM, Geohegan DB and Venton BJ, Analytical chemistry, 2015, 88, 645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lugo-Morales LZ, Loziuk PL, Corder AK, Toups JV, Roberts JG, McCaffrey KA and Sombers LA, Analytical chemistry, 2013, 85, 8780–8786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith SK, Lugo-Morales LZ, Tang C, Gosrani SP, Lee CA, Roberts JG, Morton SW, McCarty GS, Khan SA and Sombers LA, ChemPhysChem, 2018, 19, 1197–1204. [DOI] [PubMed] [Google Scholar]
- 51.Donahue CE, Miller DR, Beger TW, Johann TW and Keithley RB, Analytical Methods, 2018, 10, 1565–1576. [Google Scholar]
- 52.Borman RP, Wang Y, Nguyen MD, Ganesana M, Lee ST and Venton BJ, ACS chemical neuroscience, 2016, 8, 386–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilson LR, Panda S, Schmidt AC and Sombers LA, Analytical chemistry, 2017, 90, 888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meunier CJ, Mitchell EC, Roberts JG, Toups JV, McCarty GS and Sombers LA, Analytical chemistry, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roberts JG, Voinov MA, Schmidt AC, Smirnova TI and Sombers LA, Journal of the American Chemical Society, 2016, 138, 2516–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zestos AG, Nguyen MD, Poe BL, Jacobs CB and Venton BJ, Sensors and Actuators B: Chemical, 2013, 182, 652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


