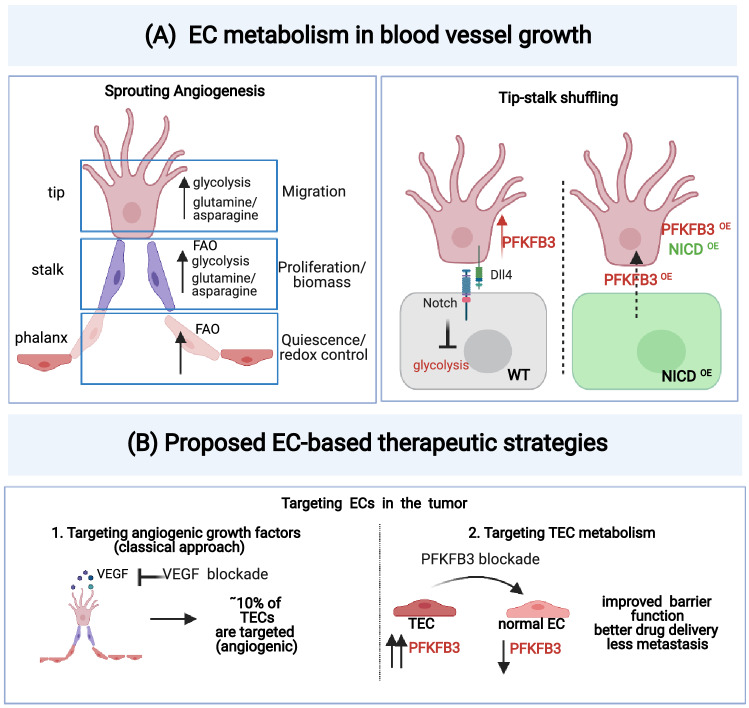
Fig. 4.
EC metabolism and proposed new EC-targeted therapies: A EC metabolism during sprouting angiogenesis: Left: Key metabolic pathways associated with each EC phenotype (tip, stalk, and phalanx ECs) involved in sprouting angiogenesis. Glycolysis and amino acid metabolism (glutamine/asparagine) are increased in tip cells to support migration. Fatty acid oxidation (FAO), glycolysis, and amino acid metabolism (glutamine/asparagine) support biomass production in stalk cells, while in phalanx ECs high levels of FAO support the maintenance of the quiescence phenotype (partially via regulation of the redox balance). Right: Regulation of tip-stalk cell shuffling: the glycolytic activator PFKFB3 is upregulated in tip cells while Notch signaling inhibits glycolysis in stalk cells. Overexpression of the Notch intracellular domain (NICD) maintains high Notch signaling and the stalk cell phenotype. However, PFKFB3 overexpression is sufficient to turn a stalk cell into a tip cell, even in the case of parallel overexpression of NICD. Therefore, PFKFB3 signaling overrules Notch signaling and drives the tip cell phenotype, and metabolism can fuel vessel sprouting independently of angiogenic signals. B Proposed EC-based therapeutic targeting strategies in cancer: 1. Fewer than 10% of tumor ECs are targeted by classic anti-angiogenic therapy (anti-VEGF), since such therapies primarily target angiogenic ECs and not the majority of other tumor ECs, which may partly explain therapy resistance. 2. Targeting of EC metabolism has emerged as a promising approach to target (tumor) angiogenesis. Inhibition of glycolysis via PFKFB3 blockade in tumors resulted in enhanced barrier function, decreased metastasis, and improved drug delivery in animal models