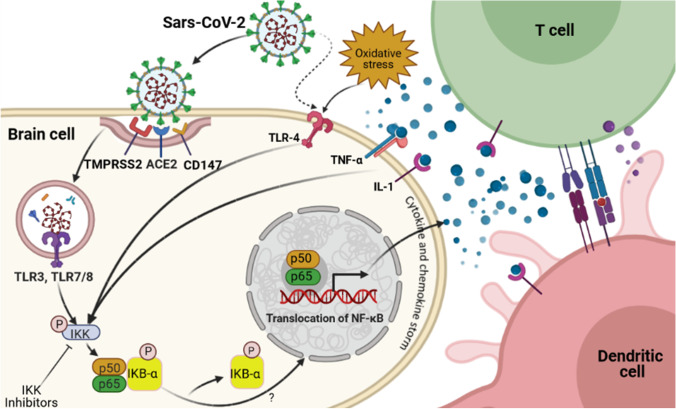
Fig. 1.
COVID infection and NF-κB signaling. This putative pathway suggests that coronaviruses are able to cause inflammation in human brain through nuclear factor kappa B (NF-κB)-dependent signaling. Binding of viral SARS-CoV to its receptors, such as CD147, angiotensin-converting enzyme 2 (ACE2), and the help of the transmembrane serine protease 2 (TMPRSS2), allows SARS-CoV to enter into host cells through cleaving/activating of viral envelope glycoproteins. Within the endosomes, viral single-stranded RNA virus activates the Toll-like receptors (TLRs), such as TLR3, TLR4, and TLR7/8. These receptors activate IKK which results in phosphorylation of the cytoplasmic inhibitor factor IκBα, which in turn leads to phosphorylation of IκBα, and subsequent degradation. As a result, NF-κB p50 and p65 are released from IκBα and translocate from the cytoplasm into the nucleus to induce transcription of various genes coding for pro-inflammatory proteins such as cytokines and chemokines. Activated NF-κB is associated with a variety of cytokine receptor- and TLR-mediated signal cascades, including binding of TNFα or IL-1 to their receptors. Excessive NF-κB activation triggers production of pro-inflammatory cytokines and a chemokine storm. T cells such as CD4 and CD8 are then activated at the site of infection by cytokines and a chemokine storm and promote further inflammation. Additionally, dendritic cells trigger adaptive immunity. This figure was developed using the BioRender online software tool