Fig. 2.
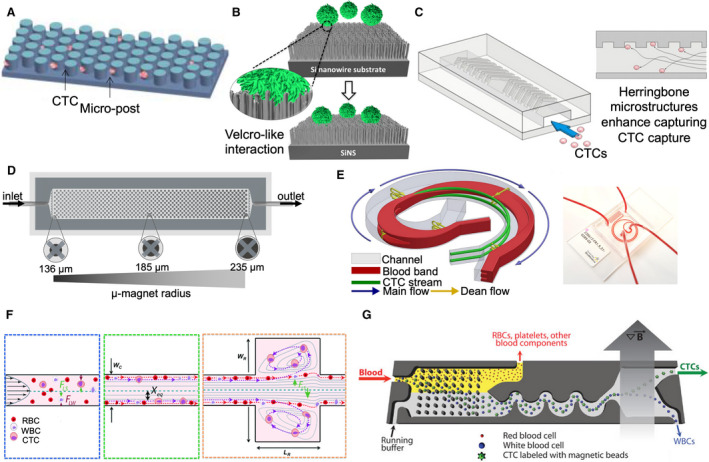
CTC enrichment approaches used for monitoring tumor resistance. (A–D) Antigen‐dependent CTC enrichment approaches. (A) Illustrative cartoon showing CTCs captured against the microposts within the CTC‐Chip. (B) The working mechanism of the nanoVelcro chip, which contains silicon nanowire substrate (SiNS) coated with EpCAM antibodies to enhance the capture efficiency of CTCs. (C) Illustration of the herringbone device (HBCTC‐Chip), featuring a microfluidic array of channels with a single inlet and outlet. (D) The microfluidic device used for magnetic ranking cytometry (MagRC) consists of 100 distinct capture zones. An array of X‐shaped microstructures creates regions of low flow velocity and circular nickel micromagnets patterned within the channel enhance the externally applied magnetic field. (E, F) Antigen‐independent CTC enrichment methods used for resistance monitoring. (E) Schematic illustration of Dean flow fractionation (DFF) principle of the antigen‐independent ClearCell Fx system (left). The target cells travel to the inner together with the blood band following the Dean flow stream in the first half curvature of the channel. Once the inner side of the channel is reached, the target cells remain focused while the blood band travels to the outer side in the second half curvature of the channel. Both target cells and nontarget cells are collected from different outlets at the end of the spiral channel. The microfluidic device utilized in the ClearCell Fx system (right) has sample and sheath inlets at one end and recovery and waste outlets at the other end. (F) Schematic of the Vortex chip featuring 8 channels. (a) At the channel inlet, the cells are randomly distributed and experience to opposing lift forces, the wall effect (F LW) and the shear‐gradient lift force (F LS). (b) As a result, the cells migrate to dynamic lateral equilibrium position (X eq), based to the channel cross section. (c) Upon entering the reservoir, the wall effect is reduced so large cells which still experience large F LW are pushed to the vortices where they become captured, whereas small cells, which do not experience sufficient F LW remain in the main flow. (G) Schematic of a hybrid CTC enrichment system, the CTC‐iChip. The blood is mixed with immunomagnetic beads and buffer before introduced into the device. CTCs are captured subsequent to inertial focusing and magnetophoresis. Figure A is reproduced with permission from Ref.[46]; Figure B is reproduced with permission from Ref. [47]; Figure C is reproduced with permission from Ref. [48]; Figure D is reproduced with permission from Ref. [41]. Figures E is reproduced with permission from Ref. [71]; Figure F is reproduced with permission from Ref. [72]; Figure G is reproduced with permission from Ref. [77]. Figure reproduced from Refs [41, 46, 47, 48, 71, 72, 77].
