Figure 2.
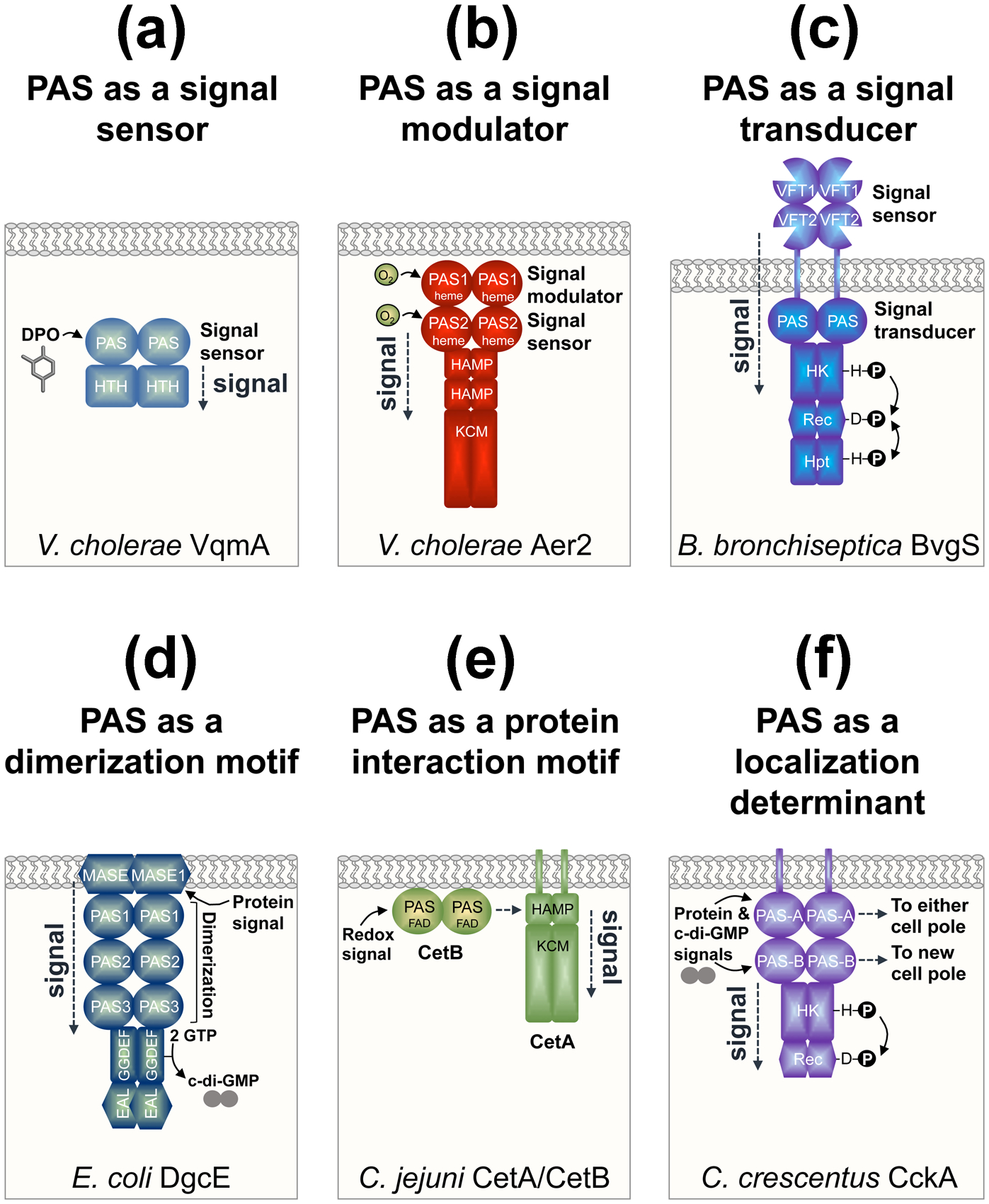
The diversity of PAS domain roles in bacterial proteins. All cartoons show the bacterial cytoplasmic membrane and the dimeric form of each protein. (a) PAS as a signal sensor as illustrated by VqmA from Vibrio cholerae. VqmA-PAS binds to the autoinducer 3,5-dimethyl-pyrazine-2-ol (DPO) and signals to a helix-turn-helix (HTH) DNA-binding domain to regulate transcription [7,8]. (b) PAS as a signal modulator as illustrated by Aer2 from V. cholerae. PAS1 modulates the extent of O2-mediated signaling from PAS2, which sends a conformational signal through two HAMP domains and a kinase control module (KCM) [15]. The KCM regulates a series of downstream chemosensory proteins. (c) PAS as a signal transducer as illustrated by BvgS from Bordetella bronchiseptica. BvgS transduces signals from a periplasmic Venus fly-trap (VFT) domain via a cytoplasmic PAS domain to C-terminal histidine kinase (HK; comprised of DHp and CA subdomains), response regulator (Rec), and histidine phosphotransfer (Hpt) domains, thus regulating kinase and phosphatase functions [16]. (d) PAS as a dimerization motif as illustrated by DgcE from Escherichia coli. The three DgcE PAS domains are essential for protein dimerization, which activates DgcE’s GGDEF (diguanylate cyclase) domain to produce c-di-GMP, and overcomes the anti-oligomerizing effect of the EAL (phosphodiesterase) domain [18]. PAS dimerization occurs after a protein complex binds to the membrane-associated sensor (MASE1) domain of DgcE. The EAL domain is degenerate and does not degrade c-di-GMP to guanosine monophosphate (GMP). (e) PAS as a protein interaction motif as illustrated by CetA and CetB from Camplyobacter jejuni. CetA and CetB constitute a bipartite energy-taxis system with the domains of the aerotaxis receptor, Aer, divided between two proteins. CetB is a PAS domain that is predicted to bind FAD, sense redox, and transmit signals through interaction with the HAMP domain of CetA [19]. (f) PAS as a cellular localization determinant as illustrated by CckA from Caulobacter crescentus. PAS-A directs CckA to either cell pole, whereas PAS-B targets CckA to new cell poles [20]. PAS-A and PAS-B also have sensory roles that regulate CckA’s kinase (HK; comprised of DHp and CA subdomains) and phosphatase (Rec) functions.
