Figure 3. Structure of the PI4KIIIα complex at the PM.
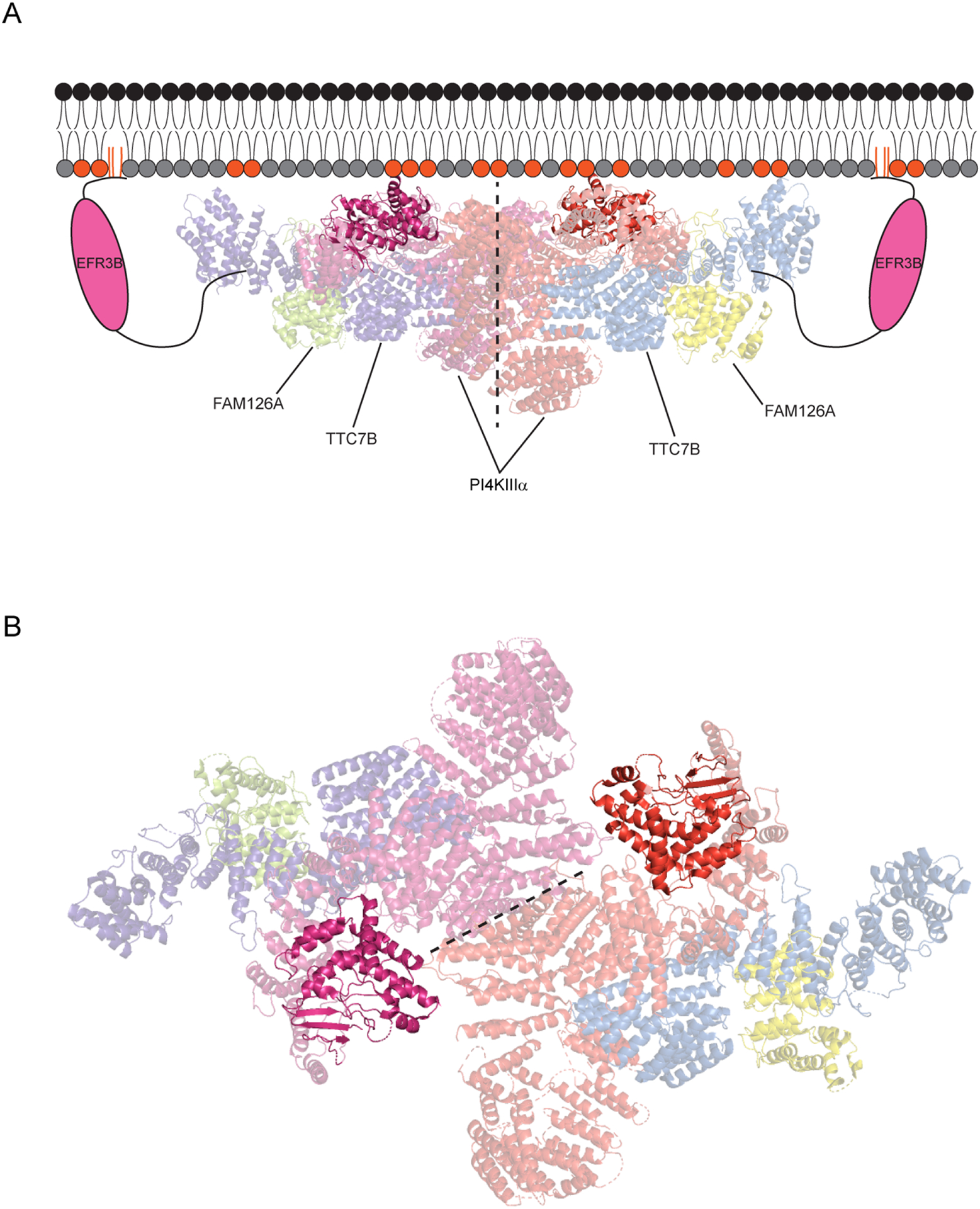
(A) EFR3B anchors the complex at the PM and recruits an ~800 kDa, hexameric complex involving a dimer of TTC7B/FAM126A/PI4KIIIα heterotrimers (~1 MDa with two molecules of EFR3B included). The kinase and TTC7B engage in stabilizing electrostatic interactions with negatively charged lipids in the inner leaflet of the PM (indicated with colored head groups). The dimerization interface between the kinases is shown with a dotted line, and the active site of each PI4KIIIα molecule is highlighted, to show its proximity to the membrane. (B) A view of the PI4KIIIα complex looking down from the plasma membrane with the kinase domains highlighted. The kinase dimerization interface is represented by a dotted line and the PI4KIIIα active sites are highlighted.
