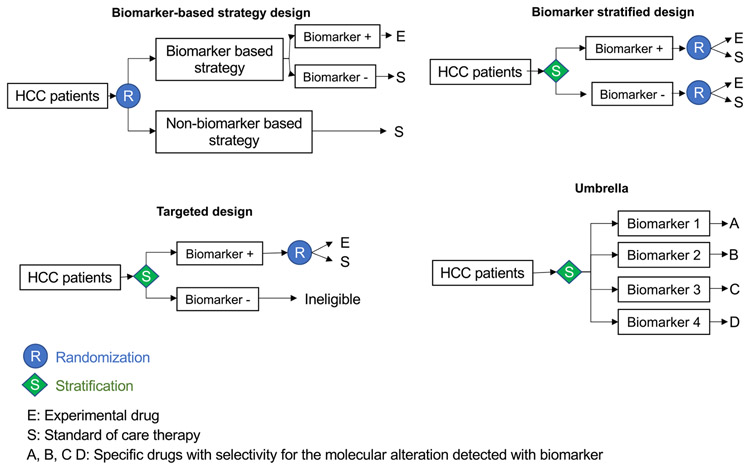
Figure 4.
Biomarker-based clinical trial designs testing new therapies. Depiction of the 4 most frequently used clinical trial designs to test the utility of biomarkers as a selection tool for new therapies. In the biomarker-based strategy, patients are randomized to a biomarker-based strategy or no stratification before they are allocated to the treatment arm. In the biomarker-stratified design, patients are stratified based on the biomarker and then randomized to receive the experimental drug or placebo. Both, patients with the biomarker and those without are included in the study. In the targeted design, only the patients with the biomarker are randomized to the drug trial (eg, trial of ramucirumab in patients with high AFP levels56). The umbrella trial design allocates specific therapies based on predicted molecular alterations detected with the biomarker, which generally includes an array of different molecular alterations.