Figure 5.
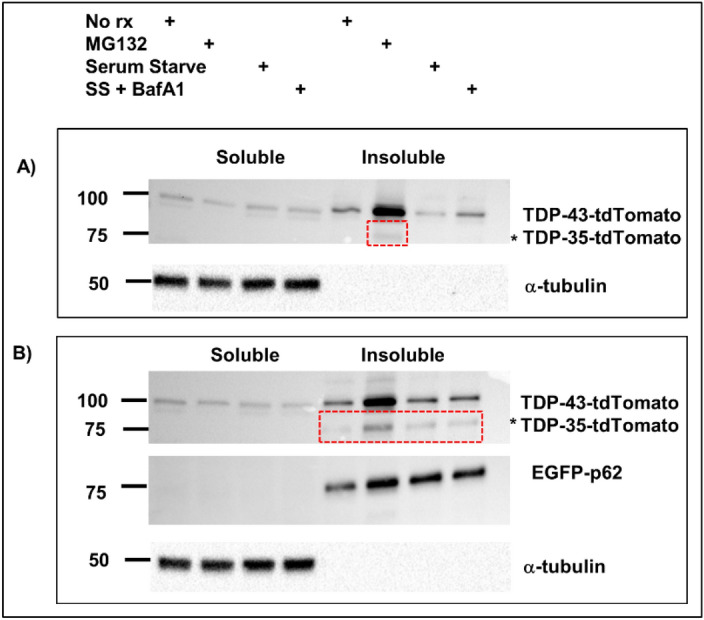
p62 creates a cleaved TDP-43 species that is also induced by proteasomal inhibition. Cells were transfected with TDP-43-tdTomato and either pcDNA3.1 (A) or EGFP-p62 (B). Cells were treated with proteasome inhibitor MG132, serum starved, serum starved with Bafilomycin A1 or left untreated. Cells were lysed and soluble and insoluble fractions obtained. Western blots were performed as indicated. Red dashed boxes indicate the presence of a *TDP-43-tdTomato cleavage product (TDP-35-tdTomato). The TDP-35 band from pcDNA3.1 transfected MG132-treated cells and EGFP-p62 non-treated cells was excised, trypsin-digested and analysed by mass spectrometry (Fig. 6). However, we did observe that the presumed TDP-35 fragment induced by EGFP-p62WT overexpression was also present in the lysates from EGFP expressing cells that were treated with the MG132. These protein bands were excised for LC–MS/MS analysis and we identified four tryptic peptides to confirm that the band matching 90-kDa TDP-tdTomato was indeed a cleavage product of TDP-43 (Fig. 6).
