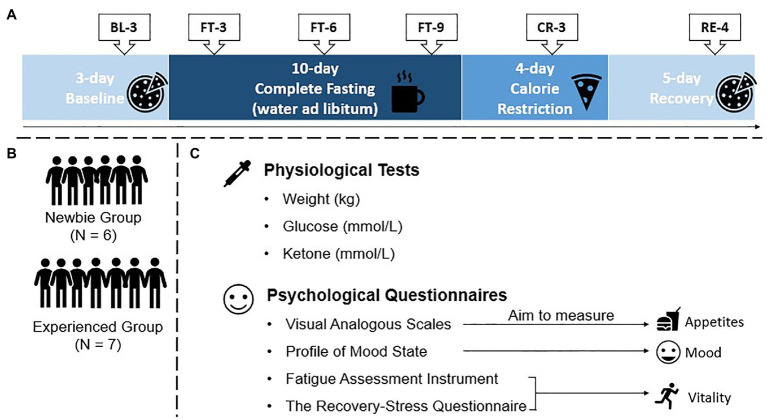
Figure 1.
Protocol of the 22-day experiment, test points, groups, and materials. (A) Protocol and test points (BL-3: 3rd day of Baseline; FT-3, FT-6, FT-9: 3rd, 6th, and 9th days of Complete Fasting, respectively; CR-3: 3rd day of Calorie Restriction; and RE-4: 4th day of Recovery). Participants were subject to no food intake limitations in the Baseline and Recovery sessions, they could drink water without solid food intake during the Complete Fasting session, and they could obtain limited food from the researchers during the Calorie Restriction session. (B) Two groups involved in the current experiment. (C) Physiological and psychological tests used in the current experiment for all six test points.