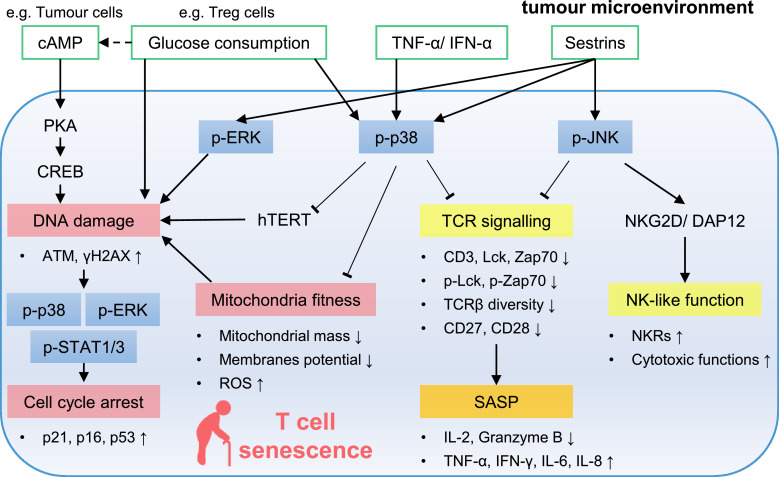
Figure 1.
Signalling pathways involved in T cell senescence in the tumour microenvironment. In senescent T cells, MAPKs can be activated by DNA damage, metabolic disorders, proinflammatory cytokines, and sestrins. The activation of p38 inhibits telomerase activity and destroys mitochondria fitness which leads to DNA damage. DNA damage further activates p38, ERK, and STAT1/3 to pronounce the expression of CKIs to prevent T cell proliferation. P-p38 and p-JNK inhibit TCR signaling to form T cell specific SASPs. P-JNK also help senescent T cells gain innate-like killing capacity. ATM, ataxia-telangiectasia mutated; cAMP, cyclic adenosine monophosphate; CKIs, cyclin-dependent kinase inhibitors; CREB, cAMP response element-binding protein; ERK, extracellular signal-regulated protein kinase; hTERT, human telomerase reverse transcriptase; IFN-γ, interferon gamma; JNK, c-Jun N-terminal kinase; MAPKs, mitogen-activated protein kinases; NK, natural killer; NKRs, natural killer like receptors; PKA, phosphorylase kinase A; ROS, reactive oxygen species; SASP, senescence-associated secretory phenotype; STAT, signal transducer and activator of transcription; TCR, T-cell receptor; TNF-α, tumour necrosis factor alpha; Tregs, regulatory T cells; γH2AX, phosphorylated H2AX