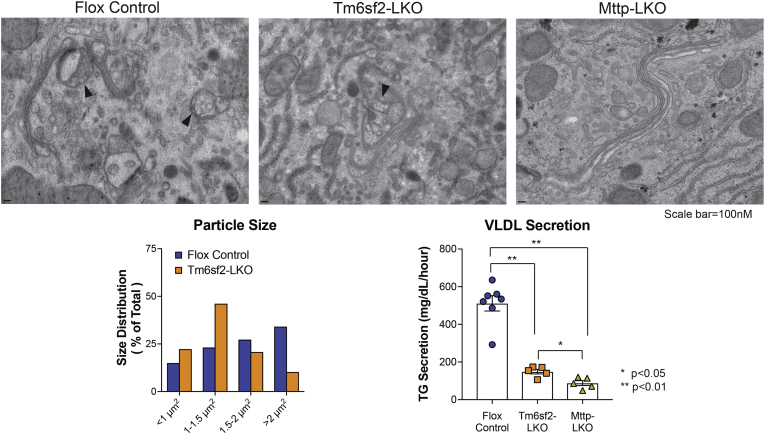
Hepatic VLDL assembly is proposed as a two-step process in which a small primordial lipoprotein particle is formed in the smooth ER by cotranslational lipidation of apolipoprotein B (APOB) by the lipid transfer chaperone microsomal triglyceride transfer protein (MTTP) (1). The nascent VLDL particles are progressively lipidated (step 2) through fusion with ER lipid droplets in a process that requires MTTP and other players, including TM6SF2 (1). Because MTTP is required for both step 1 and step 2, mice with liver-specific deletion of Mttp (Mttp-LKO) develop hepatic steatosis and complete abrogation of VLDL and APOB secretion (2). Both germline (3) and liver-specific Tm6sf2 deletor mice (Tm6sf2-LKO, Tm6sf2f/f Albumin CreTg) (4) exhibit steatosis and reduced VLDL triglyceride, but no change in APOB secretion (even in an APOB100-only background (4)), consistent with secretion of small and underlipidated VLDL particles in Tm6-LKO mice (3, 4). We used transmission electron microscopy to visualize intracellular and nascent lipoprotein particles in the ER and Golgi of Mttp-LKO, Tm6sf2-LKO, and Flox control mice. Lipoprotein particles, which appear translucent in the absence of imidazole-buffered osmium staining (2), were observed in Golgi of control and Tm6sf2-LKO mice (arrowheads, left and middle panels), but none were detected in the ER or Golgi of Mttp-LKO mice, with Golgi stacks appearing either flat or dilated, with no distinct structures inside (right). Nascent VLDL particles in Tm6sf2-LKO Golgi were smaller compared with controls (lower left, n = 68–74 particles/genotype; ImageJ software; NIH). Relative VLDL secretion rate is shown for each genotype (lower right).
EQUIPMENT: Leica EM UC7 ultramicrotome (Leica-Microsystems, Vienna, Austria) and JEM-1400 transmission electron microscopy (JEOL, Peabody, MD) with an AMT XR111 8 Megapixel scintillated CCD camera.
METHODS: Livers were perfused in situ (2.5% glutaraldehyde, 2% paraformaldehyde, 2 mM CaCl2 in 0.15 M cacodylate buffer, 5 min), excised, and fixed overnight in fresh fixative. The tissue was postfixed (1% osmium tetroxide and 0.3% potassium ferrocyanide in 0.15 M cacodylate), washed, and stained en bloc with 2% aqueous uranyl acetate (overnight, 4°C). Tissues were dehydrated in 30%, 50%, 70%, and 100% (3×) ethanol, infiltrated in a Spurr's resin/ethanol-graded series, embedded in 100% Spurr's resin, and polymerized at 60°C for 48 h. About 70 nm ultrathin sections were picked up on copper formvar/carbon support film grids and poststained with 1% tannic acid (2 min), 1% osmium tetroxide (2 min), 2% uranyl acetate (15 min), and Sato's lead for 2 min. Serum triglyceride was measured at 0 and 4 h after injection with Pluronic F-127 (10 μl/g body weight) to examine VLDL secretion.
Acknowledgments
Author contributions
E. P. N. conceptualization, investigation, and validation; G. W. S. methodology and validation; J. A .J. F. methodology, validation, supervision, and funding acquisition; and N. O. D. conceptualization, writing, review and editing, and funding acquisition.
Funding and additional information
G. W. S. and J. A. J. F. are supported by the Washington University Center for Cellular Imaging, which is funded in part by Washington University School of Medicine, The Children's Discovery Institute of Washington University, and St. Louis Children's Hospital (CDI-CORE-2015-505 and CDI-CORE-2019-813), the Foundation for Barnes-Jewish Hospital (3770), the Washington University Diabetes Research Center (DK020579), the Washington University Rheumatic Diseases Research Resource-based Center (AR073752), and Siteman Cancer Center of Barnes-Jewish Hospital and Washington University School of Medicine (CA091842). N.O.D. is supported by National Institutes of Health grants DK-119437, DK-112378, HL-151328, and Washington University Digestive Diseases Research Core Center P30 grant DK-52574 (Advanced imaging core).
References
- 1.Ginsberg H.N. ApoB SURFs a Ride from the ER to the Golgi. Cell Metab. 2021;33:231–233. doi: 10.1016/j.cmet.2021.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Raabe M., Veniant M.M., Sullivan M.A., Zlot C.H., Bjorkegren J., Nielsen L.B., Wong J.S., Hamilton R.L., Young S.G. Analysis of the role of microsomal triglyceride transfer protein in the liver of tissue-specific knockout mice. J. Clin. Invest. 1999;103:1287–1298. doi: 10.1172/JCI6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smagris E., Gilyard S., BasuRay S., Cohen J.C., Hobbs H.H. Inactivation of Tm6sf2, a gene defective in fatty liver disease, impairs lipidation but not secretion of very low density lipoproteins. J. Biol. Chem. 2016;291:10659–10676. doi: 10.1074/jbc.M116.719955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newberry E.P., Hall Z., Xie Y., Molitor E.A., Bayguinov P.O., Strout G.W., Fitzpatrick J.A.J., Brunt E.M., Griffin J.L., Davidson N.O. Liver specific deletion of mouse Tm6sf2 promotes steatosis, fibrosis and hepatocellular cancer. Hepatology. 2021 doi: 10.1002/hep.31771. [DOI] [PMC free article] [PubMed] [Google Scholar]