Abstract
According to the social brain hypothesis, the human brain includes a network designed for the processing of social information. This network includes several brain regions that elaborate social cues, interactions and contexts, i.e. prefrontal paracingulate and parietal cortices, amygdala, temporal lobes and the posterior superior temporal sulcus. While current literature suggests the importance of this network from both a psychological and evolutionary perspective, little is known about its neurobiological bases. Specifically, only a paucity of studies explored the neural underpinnings of constructs that are ascribed to the social brain network functioning, i.e. objective social isolation and perceived loneliness. As such, this review aimed to overview neuroimaging studies that investigated social isolation in healthy subjects. Social isolation correlated with both structural and functional alterations within the social brain network and in other regions that seem to support mentalising and social processes (i.e. hippocampus, insula, ventral striatum and cerebellum). However, results are mixed possibly due to the heterogeneity of methods and study design. Future neuroimaging studies with longitudinal designs are needed to measure the effect of social isolation in experimental v. control groups and to explore its relationship with perceived loneliness, ultimately helping to clarify the neural correlates of the social brain.
Key words: Social Brain, Social Isolation, Magnetic Resonance, Neurobiology
The human nature is thought to be rooted in its social interactions and relationships, which play a crucial role in survival and reproduction and support the development and preservation of physical and mental health (Cacioppo et al., 2014). In humans, extreme cases of social isolation can lead to the complete avoidance of social contexts including work, education and those involving significant others such as friends or relatives. This condition is known as Hikikomori syndrome or complete withdrawal, a phenomenon that in 2013 was estimated to affect at least one million Japanese (Saito and Angles, 2013). The evidence to date shows that this phenomenon is growing in both eastern and western countries, possibly influenced by cultural and environmental factors such as parental style and urbanicity (Tateno et al., 2012) with severe consequences for the national health systems (Cacioppo and Cacioppo, 2018).
Specifically, social isolation (and related loneliness) has been associated with increased mortality risk and depressive symptomatology, poorer cognitive performance and executive functioning, faster cognitive decline, increased threat sensitivity and alterations in the cardiovascular and neuroendocrine systems in healthy individuals (Cacioppo and Hawkley, 2009; Bhatti and ul Haq, 2017).
Conversely, abundant social relationships and interactions were shown to exert a protective effect against dementia, neurocognitive decline and mortality. More importantly, an active social life has been found to correlate with healthy behaviours such as adequate sleep, diet and exercise and discourage unhealthy behaviours such as excessive eating (Kiely et al., 2000; House, 2001). While the study of social isolation and loneliness from a clinical perspective is important for clinicians and psychiatrists, the investigation of their neuronal correlates is also crucial as it may help to understand the neurobiological phenotype of the Hikikomori syndrome and other psychiatric disorders of which social isolation and loneliness are core symptoms (e.g. depression and schizophrenia). Therefore, we aimed to describe neurobiological correlates of social isolation by means of neuroimaging studies in healthy controls. Due to the paucity of studies measuring social isolation with quantifiable methods (i.e. confinement), we also included the studies that explored the neurobiological correlates of (self-reported) loneliness as the latter is considered a psychological correlate of objective social isolation (Cacioppo and Cacioppo, 2018).
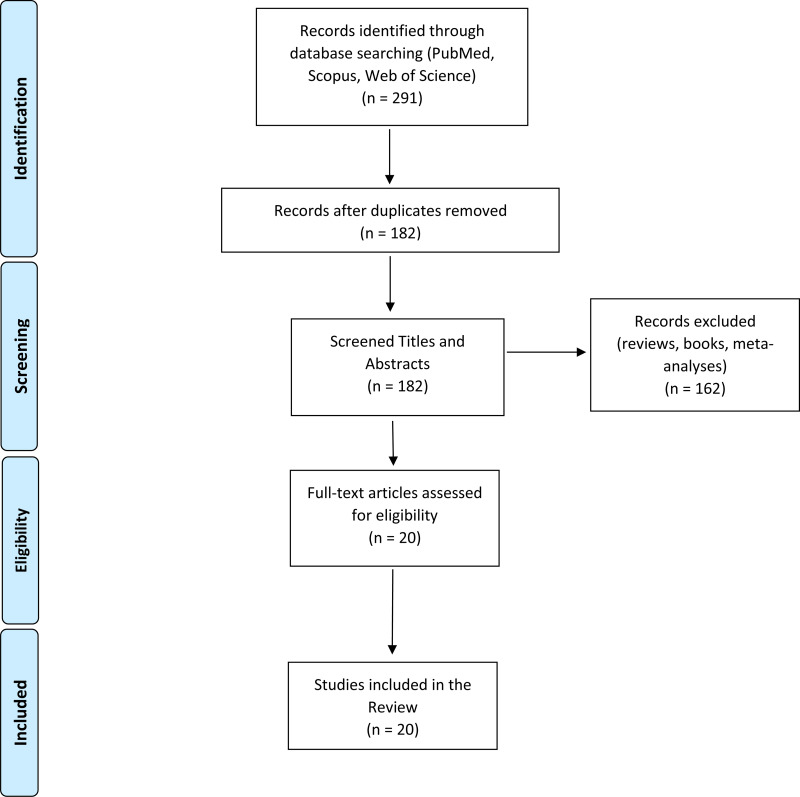
The data search was conducted through PubMed, Scopus and Web of Science databases. The following keywords were used for the search: (‘neuroimaging’ OR ‘magnetic resonance imaging’ OR ‘electroencephalography’ OR ‘Positron Emission Tomography’ OR ‘diffusion tensor imaging’) AND (‘social isolation’ OR ‘loneliness’ OR ‘hikikomori’ OR ‘complete withdrawal’). The inclusion criteria were: (i) original articles published in peer-reviewed journals between January 2000 and October 2020; (ii) English language; (iii) the inclusion of healthy participants with no psychiatric or neurologic conditions; and (iv) the application of neuroimaging technique (i.e. magnetic resonance imaging (MRI), electroencephalography (EEG), diffusion tensor imaging (DTI), positron emission tomography (PET)). Preclinical studies and case-report were excluded. A flow diagram illustrating the studies selection process is presented in Fig. 1. The literature search retrieved 182 records. After title and abstract screening, 162 articles were excluded because they clearly did not meet inclusion criteria. The remaining 20 studies were included in this review after a full-text review. Sample characteristics and neuroimaging findings from each study are shown in Table 1.
Fig. 1.
A flow diagram of the articles screening and selection process.
Table 1.
Neuroimaging studies exploring the neurobiological correlates of objective social isolation and perceived loneliness
| Reference | Study design | N participants (% F) | Age, mean (s.d.) | loneliness or social isolation assessment | Loneliness, mean (s.d.); range | Neuroimaging method | Results |
|---|---|---|---|---|---|---|---|
| Cacioppo et al. (2009) | Cross-sectional | 23 (100%) | ns | UCLA-LS | ns | fMRI |
Participants with high perceived loneliness:
|
| Kanai et al. (2012) | Cross-sectional | 108 (57%) | 23.5 (4.5) | UCLA-LS | ns | sMRI | Higher loneliness scores correlated with lower GM in the L posterior superior temporal sulcus |
| Powers et al. (2013) | Cross-sectional | 34 (64%) | 19.5 (ns) | Social exclusion manipulated experimentally | ns | fMRI | Social exclusion was associated with reduced activity in the dorsomedial PFC when viewing negative social scenes |
| Kong et al. (2015) | Cross-sectional | 308 (54%) | 19.9 (1.2) | UCLA-LS, EPQ | 41.7 (7.9); 24–62 | sMRI | Individuals with higher loneliness scores had increased GM volume in the L dorsolateral PFC |
| Nakagawa et al. (2015) | Cross-sectional | 776 (55%) | 20.7 (1.8) | UCLA-LS | 37.0 (9.2); ns | DTI | Higher loneliness scores correlated with lower WM density in the bilateral inferior parietal lobule, R anterior insula, posterior temporoparietal junction, L posterior/superior temporal sulcus, dorsomedial PFC and rostrolateral prefrontal cortex |
| Jacubowski et al. (2015) | Longitudinal | 6 (0%) | 31.3 (4.1) | Confinement (18 months) | ns | EEG | After 18 months of social isolation, participants showed decreased global cortical activity, in both α and β activity |
| Cacioppo et al. (2016) | Cross-sectional | 27 (55%) | 24 (ns) | UCLA-LS | 42.2 (11.2); 23–60 | EEG |
|
| Donovan et al. (2016) | Cross-sectional | 79 (60%) | 76.4 (6.2) | UCLA-3-LS | 5.3 (1.8); 3–10 | PiB-PET | Results indicated an association between loneliness and cortical amyloid burden in elderly adults without cognitive deficits |
| Inagaki et al. (2016) | Cross-sectional | 31 (48%) | 24.2 (7.5) | UCLA-LS | 44.2 (8.6); 33–69 | fMRI | Loneliness was associated with greater activity in the ventral striatum while viewing pictures with a social valence v. neutral picture |
| Layden et al. (2017) | Cross-sectional | 55 (56%) | 23.7 (2.1) | UCLA-LS | 40 (8.1); ns | fMRI | Higher perceived social isolation correlated with (i) greater activity in the R central operculum, R supramarginal gyrus; (ii) increased connectivity within the cingulo-opercular network, and (iii) reduced connectivity between the cingulo-opercular network and R middle/superior frontal gyrus |
| Tian et al. (2017) | Cross-sectional | 30 (0%) | 21.3 (2.4) | UCLA-LS | ns | fMRI | Higher loneliness was associated with (i) decreased blood flow from the dorsal to the ventral attentional network and from the affective to the visual network |
| Yi et al. (2018) | Cross-sectional | 100 (46%) | 28.5 (NS) | UCLA-LS | 50.5 (8.6); ns | fMRI | Higher loneliness was associated with higher amplitudes of low-frequency fluctuations activity in the inferior temporal gyrus |
| Uquillas et al. (2018) | Cross-sectional | 117 (69%) | 76 (6.2) | UCLA-3-LS | 5.19 (1.95); 3–12 | PET | Higher loneliness scores were associated with higher tau pathology in the R entorhinal cortex and R fusiform gyrus. |
| D'Agostino et al. (2019) | Cross-sectional | 50 (52%) | 62.9 (6.1) | UCLA-LS | 43.5 (8.9); ns | fMRI | No association between loneliness scores and ventral striatum and amygdala activity were found |
| Düzel et al. (2019) | Cross-sectional | 319 (51%) | 70.1 (3.7) | UCLA-LS | 1.5 (0.6); ns | sMRI | Results showed a negative association between loneliness scores and GM volumes in the L amygdala, L anterior hippocampus, L posterior para-hippocampus and L cerebellum |
| Weber et al. (2019) | Longitudinal | 33 (43%) (16 isolated; 17 not isolated) | 36.3 (7.2) | Confinement (30 days) | ns | EEG | Isolation did not affect cognitive performance nor BDNF factor or mood when comparing participants with vs. without isolation, but EEG revealed the presence of decreased activity in the parietal cortex |
| Feng et al. (2019) | Cross-sectional | 75 (13%) | 21.8 (3) | UCLA-LS | ns | fMRI | Applying a machine learning algorithm, participants' loneliness scores were predicted by within- and between-network connectivity of prefrontal, limbic and temporal systems (i.e. dorsolateral PFC, lateral orbitofrontal cortex, ventromedial PFC, caudate, amygdala) |
| Mwilambwe-Tshilobo et al. (2019) | Cross-sectional | 942 (53%) | 28 (3.4) | Loneliness survey | 50.9 (8.5); 37–82 | fMRI | Loneliness was associated with fewer modular, connections between default, frontoparietal, attention and perceptual networks |
| Wong et al. (2019) | Cross-sectional | 99 (51%) | 28.6 (17.7) | UCLA-LS; Lubben social network scale | 43 (7.7); ns | sMRI/fMRI |
|
| Stahn et al. (2019) | Longitudinal | 18 (44%) (9 isolated; 9 not isolated) | (ns) | Polar expedition (18 months) | ns | sMRI | After 18 months, isolated participants showed reduced GM volumes of the hippocampus (dentate gyrus) |
BDNF, brain-derived neurotrophic factor; EEG, electroencephalogram; EPQ, Eysenck Personality Questionnaire; fMRI, functional magnetic resonance imaging; GM, grey matter; L, left; ns, not specified; PET, positron emission tomography; PFC, prefrontal cortex; PiB, Pittsburgh compound B; R, right; sMRI, structural magnetic resonance imaging; UCLA-LS, The University of California Los Angeles, Loneliness Scale; UCLA-3-LS, 3-item version UCLA-LS; vs., versus; WM, white matter.
Seventy per cent of the studies (14 out of 20) explored the neurofunctional correlates of social isolation or loneliness with either fMRI, EEG or PET, while the remaining 30% investigated the neurostructural correlates of those constructs (i.e. grey matter (GM) volumes and white matter (WM) integrity) through structural MRI and DTI.
The University of California Los Angeles Loneliness Scale (UCLA-LS) was used in 16 out of the 20 studies (76%), to measure the feeling of loneliness. The UCLA-LS is a self-report instrument and consists of 20 items measuring general loneliness and satisfaction with social relationships. Participants are instructed to indicate how often they feel the way described by each item on a Likert scale (1 = never, 2 = rarely, 3 = sometimes, 4 = always).
As shown in Table 1, the extent of loneliness varied across studies. Specifically, the mean UCLA score was available in 12 out of 20 studies and ranged between 23 and 69 corresponding, respectively, to a low-to-high degree of loneliness, with the mean UCLA values ranging between 40 and 44 (moderate loneliness). Other studies however did not report the loneliness scores of their samples or measured objective isolation instead (confinements, polar expeditions).
Studies investigating the association between loneliness and structural brain correlates showed mixed findings. Specifically, higher loneliness scores correlated with reduced GM volumes in the posterior superior temporal sulcus, amygdala, hippocampus/para-hippocampus and cerebellum (Kanai et al., 2012; Düzel et al., 2019); increased GM volumes in the dorsolateral prefrontal cortex (PFC) (Kong et al., 2015); and lower WM density in the bilateral inferior parietal lobule, right insula, posterior temporoparietal junction, posterior superior temporal sulcus, dorsomedial and rostro-lateral PFC (Nakagawa et al., 2015). Moreover, increased GM cerebellar volumes were observed when comparing individuals with v. without susceptibility to loneliness (Wong et al., 2019). Lastly, Stahn et al. (2019) conducted a longitudinal study to explore the effects of prolonged isolation on structural brain indices and found that after 14 months of isolation, the experimental group (i.e. polar expeditioners) showed lower hippocampal volumes compared to a control group matched by age, sex and baseline hippocampal volume (Stahn et al., 2019).
Studies investigating the association between loneliness and functional brain correlates analysed the blood oxygenation level-dependant (BOLD) or EEG signals of the samples. Overall, loneliness was associated with different processing of pleasant and unpleasant social stimuli and words with affective value (Cacioppo et al., 2009, 2016; Inagaki et al., 2016; Wong et al., 2019). Specifically, Cacioppo et al. showed that, while viewing pleasant and unpleasant pictures, participants with high v. low perceived loneliness (as measured by the UCLA-LS) had reduced activations of the ventral striatum and the temporoparietal junction and greater activation of the visual cortex (Cacioppo et al., 2009). The same authors found also that images depicting social threat stimuli were differentiated more quickly by individuals with higher v. lower loneliness scores and were associated with greater activations in the PFC, visual cortex, temporal gyrus, hippocampus and supramarginal gyrus, only in lonely individuals (Cacioppo et al., 2016). Similarly, Wong et al. (2019) observed that loneliness level was positively associated with the right posterior cerebellar functional connectivity with the visual and premotor cortices when processing words with positive v. neutral affective value (Wong et al., 2019). Lastly, Powers et al. (2013) manipulated experimentally the feeling of social exclusion perceived by the participants by asking them to complete a personality questionnaire and providing them with false feedback randomly assigned. Then, participants underwent an fMRI exam while viewing pictures with affective value. Participants in which social exclusion was experimentally induced showed lower PFC activations when viewing negative social scenes (Powers et al., 2013). Conversely, D'Agostino et al. (2019) found no association between loneliness scores and brain activations during an fMRI task showing participants pictures depicting pleasant social stimuli of strangers (D'Agostino et al., 2019). This finding contrasted with previous studies reporting decreased ventral striatum activity as a function of loneliness during a similar task (Cacioppo et al., 2009). The authors suggested that discrepancies with previous studies and the absence of any association between loneliness and different processing of social cues might have been due to differences in sample size and stimuli presented.
Other studies examined the BOLD activity of the brain at rest through resting-state functional MRI and showed that social isolation and perceived loneliness were associated with increased activity in the right central operculum, right supramarginal gyrus and between default, frontoparietal, attention and perceptual networks (Layden et al., 2017; Mwilambwe-Tshilobo et al., 2019). Two studies found that higher loneliness scores were associated with (i) decreased blood flow from the dorsal to the ventral attentional network, (ii) decreased flow from the affective to the visual network and (iii) low-frequency fluctuations of the activity in the inferior temporal gyrus (Tian et al., 2017; Yi et al., 2018). In line with these results, Feng et al. (2019) applied a machine learning discrimination algorithm to the resting-state activations of healthy participants and showed that individual loneliness scores could be predicted by the connectivity of the prefrontal, limbic and temporal networks (Feng et al., 2019). Specifically, key nodes contributing to the discrimination process were in the PFC, lateral orbitofrontal cortex (OFC), caudate, amygdala and temporal regions.
Finally, prolonged objective isolation (i.e. confinement) was associated with decreased global and parietal cortical activity when measured through EEG (Jacubowski et al., 2015; Weber et al., 2019). Specifically, Jacubowski et al. (2015) evaluated the impact of the stress caused by isolation in a group of subjects that lived in confinement for 520 days. While isolated, participants were instructed to engage in physical training for 30 min/day. EEG data were collected before and after each exercise session every 2 weeks, cortisol was collected every 2 months. Results indicated decreased global cortical activity (i.e. α and β activity) and increased salivary cortisol level throughout the isolation period, thus indicating a potential stressful effect of isolation on the brain (Jacubowski et al., 2015).
Lastly, only two studies investigated the neurobiological correlates of loneliness through PET imaging and found that perceived loneliness was associated with increased amyloid and tau proteins, aggregates commonly associated with the development of several neurocognitive diseases (Donovan et al., 2016; Uquillas et al., 2018).
To summarise, current literature indicates that perceived loneliness and objective social isolation were associated with structural and functional changes in several brain regions including prefrontal, temporal and parietal cortices, limbic structures (i.e. hippocampus, amygdala, insula), the striatum and the cerebellum. Part of these brain regions belongs to the social brain network, a complex neural circuit that elaborates social cues, interactions and contexts (Dunbar, 2009). Specifically, the network encompasses the OFC, the medial PFC, the para-cingulate cortices, the amygdala, the temporal lobes and the posterior superior temporal sulcus (Frith, 2007; Adolphs, 2009).
The OFC and medial PFC are known to be involved in the monitoring of social behaviours and mentalising processes (Beer et al., 2006). For instance, patients with structural OFC alterations show a poor control of emotions and decision-making processes (Bellani and Brambilla, 2008). Similarly, the medial PFC seems to be involved in the understanding and decoding of others' behaviours in terms of mental states and social interactions (Iacoboni et al., 2004). Also, the anterior cingulate cortex has been suggested to play a role in the processing of social information (Apps et al., 2016). The role of the amygdala in social cognition is well known as its activity has been widely associated with the processing of emotions, prejudice, social judgements and behaviours (Adolphs and Spezio, 2006). Lastly, temporal lobes and the posterior superior temporal sulcus have been extensively studied in the context of the social brain as their alterations are frequently associated with autism spectrum disorders, a condition in which social cognition is severely compromised (Saitovitch et al., 2012). Particularly, the posterior superior temporal sulcus and the temporoparietal junction have been associated with the detection and processing of eye movements (Pelphrey et al., 2005).
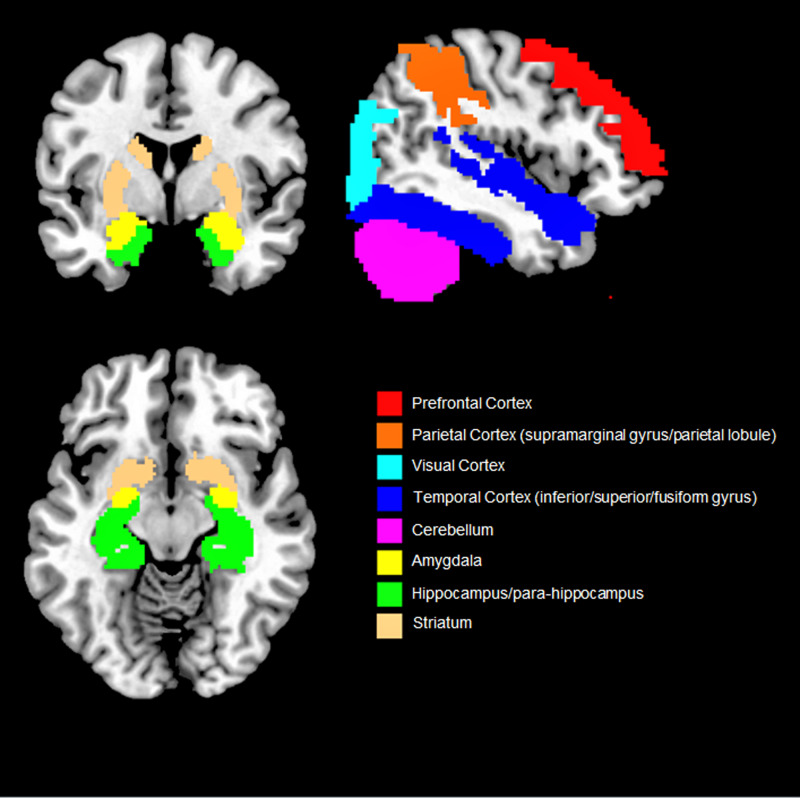
Overall, our findings suggest that objective social isolation and perceived loneliness are associated with morpho-functional changes that extend both in and out of the social brain network (i.e. PFC, temporal sulcus, amygdala and temporal cortex, parietal cortex and hippocampus) (Fig. 2). Even though some of the brain regions associated with social isolation and perceived loneliness (e.g. parietal lobule and cortex, hippocampus, cerebellum) were not included in the early theorisation of the human social brain, recent evidence suggests that they also play a role in social cognition (Rizzolatti and Fabbri-Destro, 2008; Laurita and Spreng, 2017). For example, the parietal cortex is home to the mirror neurons system, a complex network allowing primates and humans to understand the intention behind an observed motor act (Rizzolatti and Fabbri-Destro, 2008) while the hippocampus is not only involved in recalling and storing memories, but also in social navigation, namely the formation of dynamical social relationships including social distances, hierarchies and social bonds (Laurita and Spreng, 2017). Lastly, the cerebellum has been suggested to provide a supportive role in social cognition and in higher abstraction mentalising processes (Van Overwalle et al., 2014). As such, it could be speculated that social cognition (and related disorders) relies on a diffused brain circuit, broader than the social brain network, in which different regions play a central or supplementary role (Bellani and Brambilla, 2008; Crippa et al., 2016). However, results are still sparse and need replication.
Fig. 2.
Overview of the brain regions more consistently found associated with social isolation and loneliness. Only results that were reported by two or more studies are shown.
A number of limitations should be considered when interpreting the results. First, 85% of the studies were cross-sectional and measured the correlation between self-reported loneliness and brain indices while only three studies had a longitudinal design and could measure the effect of objective isolation on the brain. Moreover, the magnitude and the precision of the observed effects remain ambiguous as none of the studies reported the effect size or the confidence intervals of the results. This, in turn, made it difficult to compare the studies with each other, precluding the generalisability of the findings. Therefore, this review highlights the need to conduct future neuroimaging studies by using longitudinal designs and more quantifiable measures of social isolation (e.g. confinement). This could help clarifying the neurobiological signature of the Hikikomori syndrome and other psychiatric disorders of which social isolation and loneliness are core symptoms (e.g. depression and schizophrenia) and, ultimately, inform future diagnostic system and tailored treatments.
Acknowledgements
None.
Financial support
MGR and PB were partially supported by grants from the Ministry of Health (RF-2016-02364582).
Data
The data that support the findings of this study (search query) are available on request from the corresponding author.
Conflict of interest
None.
References
- Adolphs R (2009) The social brain: neural basis of social knowledge. Annual Review of Psychology 60, 693–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R and Spezio M (2006) Role of the amygdala in processing visual social stimuli. Progress in Brain Research 156, 363–378. [DOI] [PubMed] [Google Scholar]
- Apps MAJ, Rushworth MFS and Chang SWC (2016) The anterior cingulate gyrus and social cognition: tracking the motivation of others. Neuron 90, 692–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer JS, John OP, Scabini D and Knight RT (2006) Orbitofrontal cortex and social behavior: integrating self-monitoring and emotion-cognition interactions. Journal of Cognitive Neuroscience 18, 871–879. [DOI] [PubMed] [Google Scholar]
- Bellani M and Brambilla P (2008) Social cognition, schizophrenia and brain imaging. Epidemiologia e Psichiatria Sociale 17, 117–119. [PubMed] [Google Scholar]
- Bhatti AB and ul Haq A (2017) The pathophysiology of perceived social isolation: effects on health and mortality. Cureus 9, e994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT and Cacioppo S (2018) The growing problem of loneliness. The Lancet 391, 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT and Hawkley LC (2009) Perceived social isolation and cognition. Trends in Cognitive Sciences 13, 447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Norris CJ, Decety J, Monteleone G and Nusbaum H (2009) In the eye of the beholder: individual differences in perceived social isolation predict regional brain activation to social stimuli. Journal of Cognitive Neuroscience 21, 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo S, Capitanio JP and Cacioppo JT (2014) Toward a neurology of loneliness. Psychological Bulletin 140, 1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo S, Bangee M, Balogh S, Cardenas-Iniguez C, Qualter P and Cacioppo JT (2016) Loneliness and implicit attention to social threat: a high-performance electrical neuroimaging study. Cognitive Neuroscience 7, 138–159. [DOI] [PubMed] [Google Scholar]
- Crippa A, Vecchio Del, Ceccarelli Busti, Nobile S, Arrigoni M and Brambilla F (2016) Cortico-Cerebellar Connectivity in Autism Spectrum Disorder: What Do We Know So Far? Front Psychiatry 7, 20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agostino AE, Kattan D and Canli T (2019) An fMRI study of loneliness in younger and older adults. Social Neuroscience 14, 136–148. [DOI] [PubMed] [Google Scholar]
- Donovan NJ, Okereke OI, Vannini P, Amariglio RE, Rentz DM, Marshall GA, Johnson KA and Sperling RA (2016) Association of higher cortical amyloid burden with loneliness in cognitively normal older adults. JAMA Psychiatry 73, 1230–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar RIM (2009) The social brain hypothesis and its implications for social evolution. Annals of Human Biology 36, 562–572. [DOI] [PubMed] [Google Scholar]
- Düzel S, Drewelies J, Gerstorf D, Demuth I, Steinhagen-Thiessen E, Lindenberger U and Kühn S (2019) Structural brain correlates of loneliness among older adults. Scientific Reports 9, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C, Wang L, Li T and Xu P (2019) Connectome-based individualized prediction of loneliness. Social Cognitive and Affective Neuroscience 14, 353–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD (2007) The social brain? Philosophical Transactions of the Royal Society B: Biological Sciences 362, 671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House JS (2001) Social isolation kills, but how and why? Psychosomatic Medicine 63, 273–274. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Lieberman MD, Knowlton BJ, Molnar-Szakacs I, Moritz M, Throop CJ and Fiske AP (2004) Watching social interactions produces dorsomedial prefrontal and medial parietal BOLD fMRI signal increases compared to a resting baseline. Neuroimage 21, 1167–1173. [DOI] [PubMed] [Google Scholar]
- Inagaki TK, Muscatell KA, Moieni M, Dutcher JM, Jevtic I, Irwin MR and Eisenberger NI (2016) Yearning for connection? Loneliness is associated with increased ventral striatum activity to close others. Social Cognitive and Affective Neuroscience 11, 1096–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacubowski A, Abeln V, Vogt T, Yi B, Choukèr A, Fomina E, Strüder HK and Schneider S (2015) The impact of long-term confinement and exercise on central and peripheral stress markers. Physiology & Behavior 152, 106–111. [DOI] [PubMed] [Google Scholar]
- Kanai R, Bahrami B, Roylance R and Rees G (2012) Online social network size is reflected in human brain structure. Proceedings of the Royal Society B: Biological Sciences 279, 1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiely DK, Simon SE, Jones RN and Morris JN (2000) The protective effect of social engagement on mortality in long-term care. Journal of the American Geriatrics Society 48, 1367–1372. [DOI] [PubMed] [Google Scholar]
- Kong X, Wei D, Li W, Cun L, Xue S, Zhang Q and Qiu J (2015) Neuroticism and extraversion mediate the association between loneliness and the dorsolateral prefrontal cortex. Experimental Brain Research 233, 157–164. [DOI] [PubMed] [Google Scholar]
- Laurita AC and Spreng RN (2017) The hippocampus and social cognition. The Hippocampus from Cells to Systems. Switzerland AG: Springer, pp. 537–558. [Google Scholar]
- Layden EA, Cacioppo JT, Cacioppo S, Cappa SF, Dodich A, Falini A and Canessa N (2017) Perceived social isolation is associated with altered functional connectivity in neural networks associated with tonic alertness and executive control. Neuroimage 145, 58–73. [DOI] [PubMed] [Google Scholar]
- Mwilambwe-Tshilobo L, Ge T, Chong M, Ferguson MA, Misic B, Burrow AL, Leahy RM and Spreng RN (2019) Loneliness and meaning in life are reflected in the intrinsic network architecture of the brain. Social Cognitive and Affective Neuroscience 14, 423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Takeuchi H, Taki Y, Nouchi R, Sekiguchi A, Kotozaki Y, Miyauchi CM, Iizuka K, Yokoyama R and Shinada T (2015) White matter structures associated with loneliness in young adults. Scientific Reports 5, 17001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, Michelich CR, Allison T and McCarthy G (2005) Functional anatomy of biological motion perception in posterior temporal cortex: an fMRI study of eye, mouth and hand movements. Cerebral Cortex 15, 1866–1876. [DOI] [PubMed] [Google Scholar]
- Powers KE, Wagner DD, Norris CJ and Heatherton TF (2013) Socially excluded individuals fail to recruit medial prefrontal cortex for negative social scenes. Social Cognitive and Affective Neuroscience 8, 151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G and Fabbri-Destro M (2008) The mirror system and its role in social cognition. Current Opinion in Neurobiology 18, 179–184. [DOI] [PubMed] [Google Scholar]
- Saito T and Angles J (2013) Hikikomori: Adolescence Without End. USA: University of Minnesota Press. [Google Scholar]
- Saitovitch A, Bargiacchi A, Chabane N, Brunelle F, Samson Y, Boddaert N and Zilbovicius M (2012) Social cognition and the superior temporal sulcus: implications in autism. Revue Neurologique 168, 762–770. [DOI] [PubMed] [Google Scholar]
- Stahn AC, Gunga HC, Kohlberg E, Gallinat J, Dinges DF and Kühn S (2019). Brain changes in response to long Antarctic expeditions. New England Journal of Medicine 381, 2273–2275. [DOI] [PubMed] [Google Scholar]
- Tateno M, Park TW, Kato TA, Umene-Nakano W and Saito T (2012) Hikikomori as a possible clinical term in psychiatry: a questionnaire survey. BMC Psychiatry 12, 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Yang L, Chen S, Guo D, Ding Z, Tam KY and Yao D (2017) Causal interactions in resting-state networks predict perceived loneliness. PLoS ONE 12, e0177443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uquillas F, Jacobs HIL, Biddle KD, Properzi M, Hanseeuw B, Schultz AP, Rentz DM, Johnson KA, Sperling RA and Donovan NJ (2018) Regional tau pathology and loneliness in cognitively normal older adults. Translational Psychiatry 8, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F, Baetens K, Mariën P and Vandekerckhove M (2014) Social cognition and the cerebellum: a meta-analysis of over 350 fMRI studies. Neuroimage 86, 554–572. [DOI] [PubMed] [Google Scholar]
- Weber J, Javelle F, Klein T, Foitschik T, Crucian B, Schneider S and Abeln V (2019) Neurophysiological, neuropsychological, and cognitive effects of 30 days of isolation. Experimental Brain Research 237, 1563–1573. [DOI] [PubMed] [Google Scholar]
- Wong NML, Shao R, Wu J, Tao J, Chen L and Lee TMC (2019) Cerebellar neural markers of susceptibility to social isolation and positive affective processing. Brain Structure and Function 224, 3339–3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi Y, Li LMW, Xiao Y, Ma J, Fan L and Dai Z (2018) Brain activity mediates the relation between emotional but not instrumental support and trait loneliness. Social Cognitive and Affective Neuroscience 13, 995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study (search query) are available on request from the corresponding author.