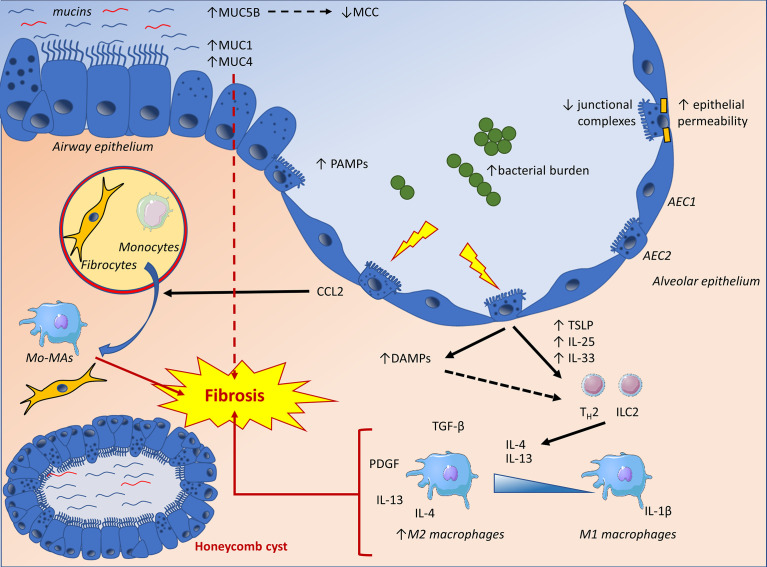
Figure 2.
The IPF lung epithelium displays increased concentrations of secreted and membrane-bound mucins, as well as altered junctional complexes, potentially influencing local barrier mechanisms and fibrosis through impaired mucociliary clearance (MCC), promotion of epithelial to mesenchymal transition (EMT) and increased epithelial permeability. Lung epithelial cells are also confronted to an increased bacterial burden and pathogen-associated molecular patterns (PAMPs). Furthermore, epithelial damage will result in the production of damage-associated molecular patterns (DAMPs), triggering pro-inflammatory pathways and TH2 polarizing cytokines. These cytokines exert a pro-fibrotic influence by directly affecting mesenchymal cells and polarizing macrophages towards an alternatively activated phenotype (M2). Finally, epithelial dysfunction will result in the release of CCL2, a chemokine directly affecting fibroblasts as well as fibrocyte recruitment and differentiation while mediating the recruitment of monocytes to the site of injury. The latter will differentiate into monocyte-derived macrophages (Mo-MA), which have been implicated in lung fibrosis. AEC1, alveolar type-1 epithelial cell; AEC2, alveolar type-2 epithelial cell; Mo-MA, monocyte-derived macrophage; MCC, mucociliary clearance; ILC2, type 2 innate lymphoid cell; TH2, type 2 helper T-cell.