Abstract
Objectives
This study was conducted to compare the microleakage and characteristics of the resin-tooth tissue interface between self-etch and etch-and-rinse adhesive systems after 48 hours and 3 months.
Materials and Methods
40 extracted premolar teeth were randomly divided into 2 groups: 1-step self-etch adhesive system – Optibond™ All-In-One, and 2-step etch-and-rinse adhesive system - Adper™ Single Bond 2. Both groups were subjected to 500 thermocycles (5°C–55°C) before scanning electron microscope (SEM) analysis or microleakage trial at 48-hour and 3-month time periods.
Results
SEM images showed the hybrid layer thickness, diameter, and length of resin tags of the self-etch adhesive (0.42 ± 0.14 µm; 1.49 ± 0.45 µm; 16.35 ± 14.26 µm) were smaller than those of the etch-and-rinse adhesive (4.39 ± 1.52 µm; 3.49 ± 1 µm; 52.81 ± 35.81 µm). In dentin, the microleakage scores of the 2 adhesives were not different in both time periods (48 hours/3 months). However, the microleakage score of etch-and-rinse adhesive increased significantly after 3 months (0.8 ± 0.63 and 1.9 ± 0.88, p < 0.05).
Conclusions
The self-etch adhesive exhibited better long-term sealing ability in dentin when compared to that of the etch-and-rinse adhesive. The greater hybrid layer thickness and dimensions of resin tags did not guarantee reliable, long-lasting sealing in the bonding area.
Keywords: Etch-and-rinse adhesive, Hybrid layer, Microleakage, Resin tags, Resin-tooth tissue interface, Self-etch adhesive
INTRODUCTION
Since Buonocore conducted the first successful bonding trial to acid-etched enamel in 1954 [1], the mechanism of bonding to dentin and enamel has been studied thoroughly and comprehensively, leading to the development of three-step adhesive systems in the early 1990s [2]. Subsequently, new generations of dental adhesive systems, such as 2-step etch-and-rinse and 1-step self-etch adhesive systems were respectively introduced [3].
The technique of using etch-and-rinse adhesive systems commences with the acid etching of the tooth tissue, followed by mandatory rinsing to remove the smear layer [4]. In enamel, the etching step dissolves enamel rods, forming a porous surface and allowing the adhesive to infiltrate the tissue by capillary action. In dentin, the etchant forms a 3–5 µm deep demineralized zone and exposes non-hydroxyapatite surrounded collagen fibers [4,5]. Self-etch adhesive systems do not require a separate etching step because acidic monomers within the adhesive can simultaneously “condition” and “prime” tooth tissue. The bonding mechanism of self-etch adhesive to tooth tissue comprises 2 aspects: micro-physical retention and chemical bonding [6]. Micro-physical retention is based on an exchange procedure in which minerals are removed and replaced by resin monomers prior to polymerization. While the micro-retention prevents an immediate debonding incident, the chemical interactions act against bonding interface degradation [5].
Cavity preparation using a high-speed handpiece leaves smear layers of 1–2 μm thickness [7]. After conditioning with 37% phosphoric acid, the smear layer and approximately 5 μm mineralized dentin beneath, are dissolved, to expose a type I collagen matrix. The space between collagen fibers is about 30 ± 11 nm where adhesive molecules penetrate up to 5 μm depth to form a hybrid layer [8]. The hybrid layer plays an important role in the longevity of the bonds. Any failure in forming the hybrid layer can lead to microleakage, which is defined as a clinically invisible pathway between the cavity wall and restorative material for the penetration of bacteria or fluids [9]. Possible symptoms of microleakage are marginal discoloration, post-operative sensitivity, secondary caries, failure of restoration, or pulp diseases.
At present, the choice of using self-etch or etch-and-rinse adhesive systems remains controversial [10,11,12]. As, in clinical practice, the self-etch adhesive systems do not require a separate acid conditioning step and moist post-rinse control, they are considered simplified adhesive materials. They offer some advantages over conventional etch-and-rinse systems, such as reduction of postoperative sensibility and less sensitive technique [13]. Numerous studies have demonstrated that self-etch and etch-and-rinse adhesive systems are equally effective in preventing microleakage [14,15,16,17]. Other studies have reported that the etch-and-rinse adhesive system is more effective in reducing microleakage [18,19]. However, few long-term studies of microleakage have been carried out.
Due to the critical nature of the hybrid layer and the potential for microleakage in clinical practice, there is a need to examine the microleakage of adhesive systems, particularly in long term, and to clarify the relationship of hybridization and characteristics of resin tags, to microleakage formation. The first purpose of the present study is to compare the microleakage in restored teeth using a self-etch adhesive system Optibond™ All-in-one (AIO) with the etch-and-rinse systems Adper™ Single Bond 2 (SB2). The second objective of the study is to examine the characteristics of the resin-tooth tissue interface in each system, and to explore its effect on potential microleakage differences.
MATERIALS AND METHODS
This in-vitro study was carried out on extracted premolar teeth of orthodontic patients after ethical assessment and approval (No. 265/ĐHYD-HĐĐĐ, University of Medicine and Pharmacy at Ho Chi Minh City). 40 teeth were collected from patients ranging in age from 16 to 40 years. Immediately after extraction, teeth were immersed in 0.5% chloramine-T solution (Pharmavet, Hanoi, Vietnam), then after the cleaning and removal of the connective tissue, the teeth were re-immersed in 0.5% chloramine-T solution and stored at a temperature of 4°C–7°C [20]. The 2 adhesive systems used in this study were a 1-step self-etch adhesive system, Optibond™ All-in-one (Kerr; Orange, CA, USA) and a 2-step etch-and-rinse adhesive system, Adper™ Single Bond 2 (3M ESPE; St. Paul, MN, USA).
The components of the 2 adhesive systems are:
• Optibond™ All-In-One: Glycerol phosphate dimethacrylate (GPDM), co-monomers, 3 kinds of nanofillers, ethanol, acetone and water, initiators.
• Adper™ Single Bond 2: Bisphenol A-glycidyl methacrylate (Bis-GMA), 2-hydroxyethyl methacrylate (HEMA), dimethacrylate Vitrebond co-polymer, nanofiller, ethanol and water, initiators.
Filling cavity preparation
Cavities were prepared on the buccal side of the teeth using carbide bur FG588 (MTD; Afula, Israel) and a high-speed handpiece under cooling water. The bur was replaced after forming every 5 cavities. The dimensions of the cavities were uniformly cut at 2 mm in width, 1.5 mm deep and 3 mm in length, across cement-enamel junctions. The depth of the cavity was identified using a periodontal probe.
Composite filling with 2 adhesive systems
The 40 teeth were equally and randomly divided into 2 groups for testing over a 48-hour and 3-month time period respectively. In each group, half of the teeth (n = 10) were restored using self-etch adhesive and the other half using etch-and-rinse adhesive. The adhesive application was followed by the manufacturer's instructions as shown in the table below (Table 1).
Table 1. Adhesives' instruction for use from manufacturers.
| Adhesive system | Etching | Rinse | Gently dry | Adhesive application | Gently dry | Curing |
|---|---|---|---|---|---|---|
| Etch-and-rinse | 15s | 10s | 5s | 15s | 5s | 10s |
| Self-etch | - | - | - | 20s × 2 times | 5s | 10s |
After photopolymerization of adhesive, Filtek™ Bulk Fill (3M ESPE) composite was delivered into the cavities and then cured using Elipar™ S10 (3M ESPE). Trial procedures were carried out for each group as follows: samples were immersed in distilled water for 24 hours or 3 months, then subjected to 500 thermocycles (5°C–55°C, 5-second dipping 25-second switching). The number of cycles was in accordance with the standard ISO/TR 11405:1994 (Dental materials - Guidance on testing of adhesion to tooth structure) which states that 500 thermal cycles are appropriate as an aging test, and the lowest acceptable number of cycles for testing long-term bonding effectiveness [21]. Following this process, teeth in each group were equally divided for scanning electron microscope (SEM) analysis, and for microleakage trial (n = 5).
Scanning electron microscope sample preparation
Roots and occlusal surfaces of the samples were cut, then sectioned using a 0.1 mm-thick disk, across the filling, then placed in resin moldings EpoxiCure™ 2 (Buehler; Lake Bluff, IL, USA). The sample surface was polished using 600, 1000 and 1500-grit sandpapers under water cooling. Samples were sonically cleaned, the surfaces conditioned for 10 seconds using 36% phosphoric acid, then rinsed under tap water for 1 minute and immersed in 5% sodium hypochlorite for 2 minutes [22].
The dehydration process was subsequently performed by dipping samples into an increasingly higher concentration of ethanol, from 25% to 50%, 75%, 95% and 100% for 20 minutes at each stage. The process was repeated 3 times at the 100% concentration stage and samples were allowed to dry at room temperature for 10 minutes [23]. Prior to SEM analysis, the surface of each sample was coated with gold in a vacuum.
SEM observation
Samples were observed under scanning electron microscope JSM-IT100 InTouchScope™ (Jeol; Akishima, Tokyo, Japan) operating at an accelerating voltage of 20 kV, at different magnifications (×500, ×2,500 and ×6,000). In the axial and gingival wall of each sample tooth, a single, random position (point) was selected for SEM analysis as the entire interface of these walls was dentin-resin. At the occlusal wall, where the interface consisted of dentin-resin and enamel-resin, 2 random positions were chosen for SEM in accordance with the interface positions.
Measurement with Adobe™ photoshop software
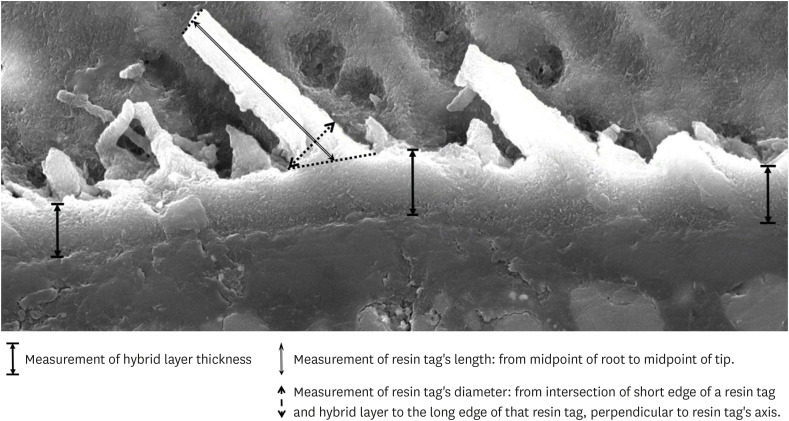
Images at ×2,500 magnification taken during SEM analysis were opened and measured in Adobe™ Photoshop (Adobe; San Jose, CA, USA). The hybrid layer thickness was identified at 3 positions on each image: at the center and both sides. Resin tag lengths were measured from the root to the tip of each tag. For the purpose of standardization, the measurements were taken from the midpoint of the root to the midpoint of the tip. Three clearly visible resin tags, with distinctly identifiable roots and tips, were selected and measured in each image. Resin tag diameters were measured at the root, from the intersection of the short edge of a resin tag and the hybrid layer, to the long edge of that resin tag, perpendicular to the resin tag's axis (Figure 1). Up to three resin tags were selected, and their diameters measured in each image. The ruler tool was utilized, and dimensions were taken using the scaled ruler embedded in each SEM image.
Figure 1. Measurement of hybrid layer, length and diameter of resin tag.
Microleakage trial preparation
All surfaces of teeth were covered by 2 layers of nail varnish except for the filling surface itself and 1 mm beyond its margin, to allow the dyeing liquid to penetrate at the interface. The dye penetration method was selected to test the sealing ability of adhesives, as the smaller size of dye particles compared to that of bacteria, would indicate, by visible penetration of the interface, early signs of a compromised margin [24]. All samples were then immersed in a 3% methylene blue solution for 24 hours before being completely cleaned under tap water for 15 minutes [25]. Among the other dye types which can be used to track microleakage, such as basic fuchsin and silver nitrate, methylene blue was chosen due to its low cost, availability and ease of use [24]. A water-cooled, 0.1 mm thick diamond disk Accutom-5 (Struers; Cleveland, OH, USA) was used to section the tooth in a buccal-lingual direction through the filling to observe dye penetration under an optical microscope (X40).
Microleakage evaluation
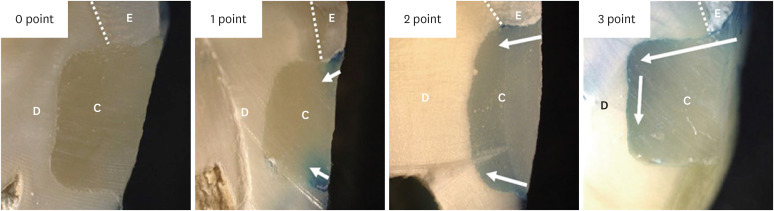
Dye penetration was observed using an optical microscope with images recorded by an integrated camera. The microleakage score was evaluated according to El Sayed's grade (Table 2 and Figure 2) [25].
Table 2. Microleakage evaluation.
| Point | Condition |
|---|---|
| 0 | No penetration |
| 1 | Dye penetrated to occlusal and gingival wall, but not excess half of cavity depth |
| 2 | Dye penetrated to occlusal and gingival wall, excess half of cavity depth |
| 3 | Dye penetrated to axial wall |
Figure 2. Microleakage score evaluation.
C, composite; D, dentin; E, enamel; Arrow, dye penetration.
Statistical analysis
The collected data were processed using IBM® SPSS® Statistics Version 23 (IBM; Armonk, NY, USA) software. The Shapiro-Wilk test was applied to identify the normality of data. The non-parametric Kruskal-Wallis and Mann-Whitney tests were respectively used to compare values among groups and between the 2 groups. The level of statistical significance was set at p < 0.05.
RESULTS
Morphology of resin-enamel interface
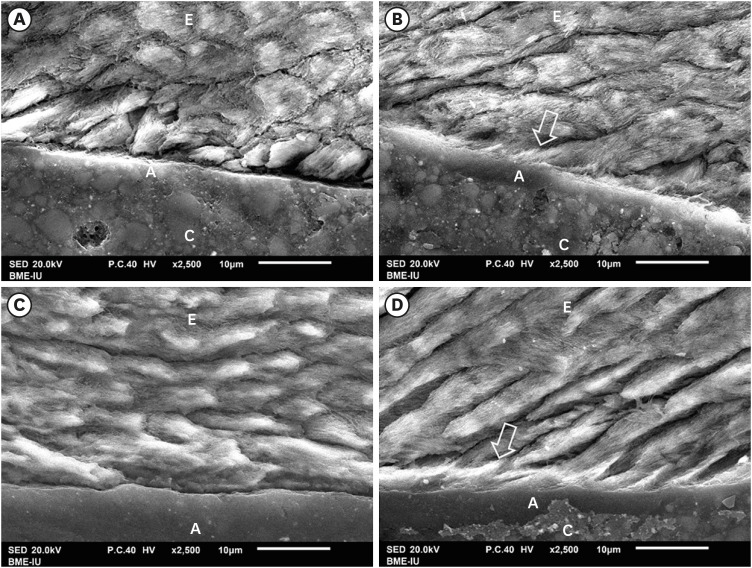
The results of SEM evaluation after 48 hours and 3 months respectively, indicated that the AIO self-etch adhesive had almost no mechanical micro-retention with tooth enamel. This was illustrated by the nearly flat resin-enamel interface and by the presence of micro-gaps (Figure 3A and 3C). In contrast, the interface between SB2 etch-and-rinse adhesive and enamel was seamless and resin tags inside enamel were clearly observed (Figure 3B and 3D).
Figure 3. SEM of resin-enamel interface. SEM image of AIO self-etch adhesive after 48 hours (A) and 3 months (C) showing almost flat enamel interface. SB2 etch-and-rinse adhesive after 48 hours (B) and 3 months (D) showing deeply infiltrated resin tags in enamel (arrow).
A, adhesive; C, composite; E, enamel.
Morphology of resin-dentin interface
The SEM findings revealed that AIO self-etch adhesive produced a thin hybrid layer with small-diameter, short and variously distributed resin tags. Notably, many resin tags were imperfectly formed (Figure 4A and 4C). The SB2 etch-and-rinse adhesive produced a thick hybrid layer with long, broad and highly dense resin tags (Figure 4B and 4D). There were many funnel-shaped resin tags with big diameters. In fact, when being randomly observed and measured, the mean hybrid layer thickness (0.42 ± 0.14 µm), mean resin tag's diameter (1.49 ± 0.45 µm) and length (16.35 ± 14.26 µm) formed by AIO self-etch adhesive showed smaller values compared to those formed by SB2 etch-and-rinse adhesive (4.39 ± 1.52 µm; 3.49 ± 1 µm; 52.81 ± 35.81 µm, respectively).
Figure 4. SEM of resin-dentin interface. The AIO self-etch adhesive (A and C) formed thin hybrid layer (between 2 black arrows) (0.42 ± 0.14 µm) with small-diameter (1.49 ± 0.45 µm), short and scattered resin tags (16.35 ± 14.26 µm), some imperfectly formed tags (white arrow). The hybrid layer of the SB2 etch-and-rinse adhesive (B and D) was thick (4.39 ± 1.52 µm) with numerous long (52.81 ± 35.81 µm), large-diameter (3.49 ± 1 µm), funnel-shaped resin tags.
A, adhesive; C, composite; D, dentin; H, hybrid layer; R, resin tag.
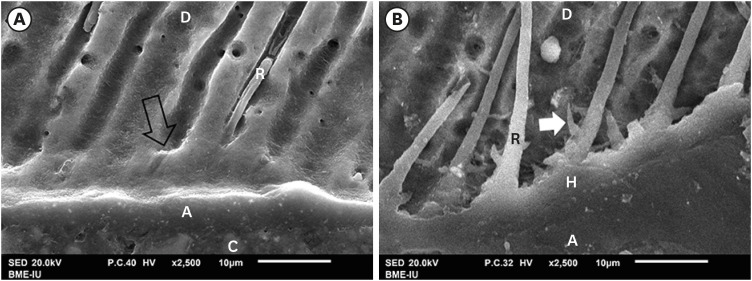
The “smear plugs” sealed the dentin tubules and subsequently reducing the permeability of AIO self-etch adhesive (Figure 5A). On the contrary, resin tags of SB2 etch-and-rinse adhesive infiltrated deeply not only inside dentin tubules, but also in the lateral branches of dentin tubules, creating the “hook” shapes (Figure 5B).
Figure 5. SEM of resin-dentin interface. SEM image of AIO self-etch adhesive (A) showing the “smear plugs” sealing dentin tubules (black arrow) and forming short resin tags. The SB2 etch-and-rinse adhesive (B) showed resin tags infiltrating deeply into dentin tubules and their lateral branches (white arrow).
A, adhesive; C, composite; D, dentin; H, hybrid layer; R, resin tag.
Microleakage formed with 2 adhesive systems
In enamel, the mean scores of the AIO self-etch adhesive were significantly higher than those of the SB2 etch-and-rinse adhesive at both 48 hours and 3 months (p < 0.05) (Table 3). In dentin, there was no significant difference in the microleakage mean score between the 2 adhesives at 48 hours or 3 months. However, after 3 months, the mean score of the SB2 etch-and-rinse adhesive in dentin increased significantly, while there was no significant change in microleakage of AIO self-etch adhesive samples (Table 4).
Table 3. Microleakage mean scores in enamel.
| Adhesive system | 48 hr | 3 mon |
|---|---|---|
| AIO Self-etch (n = 10) | 2 ± 0.94 | 2.2 ± 0.92 |
| SB2 Etch-and-Rinse (n = 10) | 1 ± 1.15 | 1 ± 0.94 |
| p | 0.039* | 0.014† |
*Significant difference of microleakage mean score in enamel between the two adhesive systems in specimens after 48 hours, Mann-Whitney test, p < 0.05; †Significant difference of microleakage mean score in enamel between the two adhesive systems in specimens after 3 months, Mann-Whitney test, p < 0.05.
Table 4. Microleakage mean scores in dentin.
| Adhesive system | 48 hr | 3 mon | p |
|---|---|---|---|
| AIO Self-etch (n = 10) | 1 ± 1.15 | 1.7 ± 1.34 | - |
| SB2 Etch-and-Rinse (n = 10) | 0.8 ± 0.63 | 1.9 ± 0.88 | 0.008* |
*Significant difference of microleakage mean score in dentin of specimens after 48 hours and 3 months with SB2 etch-and-rinse adhesive, Mann-Whitney test, p < 0.05.
DISCUSSION
Previous studies have demonstrated that a hybridized resin-dentin layer that incorporates the infiltration of resin tags into tubules and the permeation of adhesive into demineralized porous dentin, results in the mechanism bonding, sealing, and protecting dentin from permeability [26,27,28,29,30]. Therefore, the evaluation of microleakage and hybrid layer for each adhesive is very important. This study was conducted to compare the characteristics of hybrid layer, and microleakage of 2 adhesive systems in both the short and long term, and to explain the relationship between the role of hybrid layer, resin tag and microleakage at the resin-tooth tissue interface. Particularly, microleakage formation in a long period of time was rarely mentioned in previous studies.
The formation of the hybrid layer plays an important role in the dentin bonding procedure. The thickness of the hybrid layer is determined by the ability of dentin demineralization of adhesive systems. The SB2 etch-and-rinse adhesive system, with 35% phosphoric acid in the application procedure can create a thicker hybrid layer than that of the AIO self-etch adhesive. With regard to the self-etch adhesive system, the thickness of the hybrid layer is determined by how strong the acidic monomers are. Many monomers have recently been synthesized and used in dental self-etch adhesives, such as 10-methacryloxydecyl dihydrogen phosphate (10-MPD), 4-methacryloxyethyl trimellitic acid (4-MET) and 2-methacryloxyethyl phenyl hydrogen phosphate (Phenyl-P), The AIO self-etch adhesive used in our study, contains GPDM functional monomers, one of the first chemicals found to be capable of bonding with dentin [31]. Initially, GPDM was used in etch-and-rinse adhesive systems and has recently been incorporated into self-etch adhesives as a functional monomer. In spite of its early application, the chemical reaction of GPDM with hydroxyapatite was only recently described in 2018 by Yoshihara et al. [32]. The authors found that GPDM-calcium salts were highly soluble and then demineralized calcium according to adhesion–decalcification concept, similar to the characteristic of phenyl-P and 4-MET monomers. This, in conjunction with repeat adhesive applications (2 × 20 seconds applications), may explain why the AIO self-etch adhesive used in our study still formed a fairly substantial hybrid layer (0.42 ± 0.14 µm) despite its “mild” pH of 2.5, compared to that of other adhesives with higher acidity levels. Although the pH of AIO self-etch adhesive is considered mild (≈ 2.5), GPDM monomers contained within AIO have a tendency to dissolve and etch dentin. After which, a hybrid layer with a particular thickness also formed in the dentin surface.
SEM images of the AIO self-etch adhesive revealed short (16.35 ± 14.26 µm), small diameter (1.49 ± 0.45 µm), scattered resin tags. More importantly, imperfectly formed resin tags were also observed. The “mild” pH of the AIO self-etch adhesive was considered to be the reason for these results, because it could not demineralize peritubular dentin. In addition, the differing smear layer thicknesses could also affect the infiltration of adhesive deep into the dentin tubules [33]. The voids or imperfect formation of resin tags proved that the infiltration of adhesive into dentin tubules was not consistent. Incomplete evaporation of the adhesive system solvent may be another reason for the imperfect formation of resin tags. Many studies showed that incomplete evaporation could occur with dental adhesives containing ethanol or acetone solvents [33,34].
The SB2 etch-and-rinse adhesive in our study formed large diameter (3.49 ± 1 µm), funnel-shaped resin tags with many lateral branches infiltrating deeply into the dentine. The high value of mean resin tags' diameter probably resulted from the use of 37% phosphoric acid. The acid dissolved the smear layer, demineralized the dentin, and exposed the dentin collagen matrix. More importantly, it dissolved almost all of the highly mineralized peritubular dentin, resulting in wide-open and funnel-shaped dentin tubules.
SEM analysis and random measurement also showed that the mean resin tags' length formed by the AIO self-etch adhesive, were shorter than those formed by the SB2 etch-and-rinse adhesive (52.81 ± 35.81 µm). However, the large standard deviation suggested the length of resin tags was extremely variable. This could be explained by the likelihood that the observed length of resin tags completely relied on the direction of sections; one direction being favorable to the exposure of long tags, while another direction may reveal a range of shorter resin tags. Few studies have investigated resin tag length. However, those which have, identified comparable results of resin tags length formed by self-etch adhesive systems when compared to ours (12.61 µm) [35]. The long resin tags formed by the SB2 etch-and-rinse adhesive in our study could have resulted from the effect of 37% phosphoric acid, the HEMA in SB2 adhesive's composition and more particularly, the lack of pulp pressure in extracted teeth.
The purpose of using adhesive in dentistry is to increase sealing ability between the filling material and tooth tissue, to enhance restoration retention and reduce microleakage risk at the resin-tooth interface. After 48 hours, the microleakage score of AIO self-etch adhesive in enamel (2 ± 0.94) was significantly higher than that of SB2 etch-and-rinse adhesive (1 ± 1.15). After 3 months, the mean score of SB2 adhesive was still 1, while that of AIO increased to 2.2. The enamel surface treated with 35% phosphoric acid for 15 seconds had multiple deep pores facilitating the deep penetration of SB2 etch-and-rinse adhesive to form a micro-interlocking mechanism, resulting in long-term sealing ability. SEM results for the resin-enamel interfaces confirmed that the SB2 etch-and-rinse adhesive resulted in a continuous fusion between the enamel and adhesive layer, with resin tags within the enamel being clearly defined. In contrast, the AIO self-etch adhesive did not sufficiently create a micro-interlocking mechanism, demonstrated by the flat interface of enamel, separate from the adhesive layer. The self-etch adhesive system would dissolve different amounts of enamel depending on how “strong” the adhesive was. In this study, the AIO self-etch adhesive system (pH = 2.5) would not etch enamel as much as phosphoric acid did, in order to form resin infiltration images. The difference in enamel surface conditioning using self-etch and etch-and-rinse adhesive systems has been mentioned in many studies. In 2009, Moura et al. [11] concluded that enamel treated with 35% phosphoric acid for 15 seconds would create a uniform interface with multiple porosities, expose enamel rods and remove the smear layer completely. In contrast, the self-etch adhesive (pH = 2, Clearfil SE Bond) showed quite flat enamel surface with scattered, shallow porosities and a remaining smear layer. More importantly, no self-etch adhesive in the study could expose enamel rods, as 35% phosphoric acid did. In 2017, Alcantara-Galeana et al. [36] demonstrated that 37% phosphoric acid dissolved large amounts of enamel tissue, forming a traditional honeycomb etching pattern. On the other hand, the self-etching agent was more conservative, exhibiting uniformly shallow porosities.
In our study, although the enamel microleakage mean score of AIO self-etch adhesive increased after 3 months, the difference was not significant when compared to that after 48 hours. This proved that the connection between the AIO self-etch adhesive and enamel remained stable though there was no micro-interlocking mechanism. Indeed, enamel demineralization to create a porous surface was the only aspect determining the sealing ability of an adhesive system. Therefore, apart from altering the enamel surface, other factors such as viscosity, surface tension, chemical interaction of acidic monomers with enamels, and the proportion of water need to be considered when assessing sealing ability [11].
In dentin, after 48 hours, the microleakage mean score of the 2 adhesive systems was not different, although the values of the thickness of the hybrid layer and the dimensions of resin tags of AIO self-etch adhesive were lower when compared to SB2 etch-and-rinse adhesive. This proved that the quality of the hybrid layer, not its thickness, determined sealing ability. As analyzed above, GPDM, a functional monomer in AIO adhesive, was proved to decalcify dentin, forming unstable GPDM-calcium salts. [32]. Wang et al. showed in another research article that the effectiveness of GPDM to decalcify dentin, forming longer resin tags, results in an improved micro-interlocking mechanism, which contributes to an increase in sealing ability [37]. The self-etch adhesive containing GPDM used in Wang's research created higher sealing ability than other self-etch adhesives. A further advantage of GPDM was that the monomer included 2 methacrylate groups for polymerization, so it could create strong bonds to other monomers and restorative materials. A superior polymerization ability meant an increased quality of polymer matrix and improved mechanical properties. Furthermore, the manufacturer's recommendation to apply the adhesive layer twice also facilitated the etching process and penetration of adhesive.
After 3-months, the mean dentin microleakage scores increased for both adhesive groups, but there was no significant difference between the 2 groups. This showed that the sealing ability of the AIO self-etch adhesive to dentin was comparable to that of the SB2 etch-and-rinse adhesive. One remarkable result in our study was that the mean dentin microleakage score of the SB2 etch-and-rinse adhesive increased significantly after 3 months. This observation could be explained by the degradation of the hybrid layer formed with etch-and-rinse adhesive. This degradation could occur either in the resin, in the collagen matrix, or both. The degradation of the resin was due to the absorbance of water into the low cross-linking density or hydrophilic zone, because of limited conversion at these positions [38,39]. If the hydrophilic groups were not sufficiently polymerized, they would degrade quickly in a wet environment and expose the collagen matrix, which was previously covered by resin. The exposed collagen would be sensitive to proteinase. Since the quality of dentin sealing is affected by resin degradation and subsequent collagen matrix degradation, clinicians should consider solutions to inhibit these proteinases, in order to prolong bonding connection. There are many recommended solutions, such as enzyme inhibition, synthetic MMP inhibitors, collagen cross-linking, ethanol wet bonding and recently biomimetic remineralization [30].
CONCLUSIONS
Within the limitations of this in vitro study, it can be concluded that the microleakage formation with the AIO self-etch adhesive in enamel was significantly greater than with the SB2 etch-and-rinse adhesive. In dentin, the microleakage score of the 2 adhesive systems was not significantly different. The AIO self-etch adhesive system exhibited more stable dentin sealing ability after aging in the long term (3 months) than the SB2 etch-and-rinse adhesive system. The hybrid layer thickness, morphology and sizes of the resin tags do not reflect the dentin sealing ability. However, it is necessary to carry out further experiments with longer aging time, under simulated chewing force, to use more advanced methods to evaluate microleakage such as electrochemical impedance, microcomputed tomography, radioactive isotopes and optical coherence tomography, and to compare the sealing ability of traditional adhesives with current, trending, universal adhesives.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: Tran XV, Tran QK.
- Data curation: Tran XV, Tran QK.
- Formal analysis: Tran XV, Tran QK.
- Investigation: Tran QK, Tran XV.
- Methodology: Tran QK, Tran XV.
- Project administration: Tran XV, Tran QK.
- Supervision: Tran XV, Tran QK.
- Validation: Tran XV, Tran QK.
- Visualization: Tran XV, Tran QK.
- Writing - original draft: Tran XV, Tran QK.
- Writing - review & editing: Tran QK, Tran XV.
References
- 1.Buonocore MG. A simple method of increasing the adhesion of acrylic filling materials to enamel surfaces. J Dent Res. 1955;34:849–853. doi: 10.1177/00220345550340060801. [DOI] [PubMed] [Google Scholar]
- 2.Kugel G, Ferrari M. The science of bonding: from first to sixth generation. J Am Dent Assoc. 2000;131(Supplement):20S–25S. doi: 10.14219/jada.archive.2000.0398. [DOI] [PubMed] [Google Scholar]
- 3.Van Meerbeek B, Yoshihara K, Van Landuyt K, Yoshida Y, Peumans M. From Buonocore's pioneering acid-etch technique to self-adhering restoratives. A Status perspective of rapidly advancing dental adhesive technology. J Adhes Dent. 2020;22:7–34. doi: 10.3290/j.jad.a43994. [DOI] [PubMed] [Google Scholar]
- 4.Sofan E, Sofan A, Palaia G, Tenore G, Romeo U, Migliau G. Classification review of dental adhesive systems: from the IV generation to the universal type. Ann Stomatol (Roma) 2017;8:1–17. doi: 10.11138/ads/2017.8.1.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sezinando A. Looking for the ideal adhesive – a review. Rev port Estomatol Med Dent Cir Maxilofac. 2014;55:194–206. [Google Scholar]
- 6.Giannini M, Makishi P, Ayres AP, Vermelho PM, Fronza BM, Nikaido T, Tagami J. Self-etch adhesive systems: a literature review. Braz Dent J. 2015;26:3–10. doi: 10.1590/0103-6440201302442. [DOI] [PubMed] [Google Scholar]
- 7.Van Landuyt K, De Munck J, Coutinho E, Peumans M, Lambrechts P, Van Meerbeek B. Bonding to dentin: smear layer and the process of hybridization. In: Eliades G, Watts D, Eliades T, editors. Dental hard tissues and bonding. Berlin, Heidelberg: Springer; 2005. pp. 89–122. [Google Scholar]
- 8.Talungchit S, Jessop JL, Cobb DS, Qian F, Geraldeli S, Pashley DH, Armstrong SR. Ethanol-wet bonding and chlorhexidine improve resin-dentin bond durability: quantitative analysis using raman spectroscopy. J Adhes Dent. 2014;16:441–450. doi: 10.3290/j.jad.a32695. [DOI] [PubMed] [Google Scholar]
- 9.Taylor MJ, Lynch E. Microleakage. J Dent. 1992;20:3–10. doi: 10.1016/0300-5712(92)90002-t. [DOI] [PubMed] [Google Scholar]
- 10.Chen C, Niu LN, Xie H, Zhang ZY, Zhou LQ, Jiao K, Chen JH, Pashley DH, Tay FR. Bonding of universal adhesives to dentine--Old wine in new bottles? J Dent. 2015;43:525–536. doi: 10.1016/j.jdent.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Moura SK, Reis A, Pelizzaro A, Dal-Bianco K, Loguercio AD, Arana-Chavez VE, Grande RH. Bond strength and morphology of enamel using self-etching adhesive systems with different acidities. J Appl Oral Sci. 2009;17:315–325. doi: 10.1590/S1678-77572009000400009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thanaratikul B, Santiwong B, Harnirattisai C. Self-etch or etch-and-rinse mode did not affect the microshear bond strength of a universal adhesive to primary dentin. Dent Mater J. 2016;35:174–179. doi: 10.4012/dmj.2015-109. [DOI] [PubMed] [Google Scholar]
- 13.Van Meerbeek B, Yoshihara K, Yoshida Y, Mine A, De Munck J, Van Landuyt KL. State of the art of self-etch adhesives. Dent Mater. 2011;27:17–28. doi: 10.1016/j.dental.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 14.Abdalla AI, Davidson CL. Shear bond strength and microleakage of new dentin bonding systems. Am J Dent. 1993;6:295–298. [PubMed] [Google Scholar]
- 15.Yap AU, Ho KS, Wong KM. Comparison of marginal sealing ability of new generation bonding systems. J Oral Rehabil. 1998;25:666–671. doi: 10.1046/j.1365-2842.1998.00296.x. [DOI] [PubMed] [Google Scholar]
- 16.Schuldt C, Birlbauer S, Pitchika V, Crispin A, Hickel R, Kühnisch J. Shear bond strength and microleakage of a self-etching adhesive for fissure sealing after different types of aging. Dent Mater J. 2016;35:490–497. doi: 10.4012/dmj.2015-323. [DOI] [PubMed] [Google Scholar]
- 17.Mohammad ZK, Luddin N, Masudi SM, Alam MK. Microleakage in class II composite restorations bonded with self-etch, one-step, one-component adhesive system: a CLSM study. Int Med J. 1994;22:418–421. [Google Scholar]
- 18.Mauro SJ, Durão VC, Briso AL, Sundefeld ML, Rahal V. Effect of different adhesive systems on microleakage in class II composite resin restorations. Int J Adhes Adhes. 2012;34:6–10. [Google Scholar]
- 19.Waldman GL, Vaidyanathan TK, Vaidyanathan J. Microleakage and resin-to-dentin interface morphology of pre-etching versus self-etching adhesive systems. Open Dent J. 2008;2:120–125. doi: 10.2174/1874210600802010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armstrong S, Breschi L, Özcan M, Pfefferkorn F, Ferrari M, Van Meerbeek B. Academy of Dental Materials guidance on in vitro testing of dental composite bonding effectiveness to dentin/enamel using micro-tensile bond strength (μTBS) approach. Dent Mater. 2017;33:133–143. doi: 10.1016/j.dental.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 21.Gale MS, Darvell BW. Thermal cycling procedures for laboratory testing of dental restorations. J Dent. 1999;27:89–99. doi: 10.1016/s0300-5712(98)00037-2. [DOI] [PubMed] [Google Scholar]
- 22.Gateva N, Kabaktchieva R. Hybrid layer thickness in primary and permanent teeth - a comparison between total etch adhesives. J IMAB. 2012;18:191–199. [Google Scholar]
- 23.Pereira CN, Daleprane B, Barbosa PF, Moreira AN, de Magalhães CS. Qualitative evaluation of scanning electron microscopy methods in a study of the resin cement/dentine adhesive interface. Microsc Microanal. 2014;20:268–275. doi: 10.1017/S143192761301369X. [DOI] [PubMed] [Google Scholar]
- 24.Patel MU, Punia SK, Bhat S, Singh G, Bhargava R, Goyal P, Oza S, Raiyani CM. An in vitro evaluation of microleakage of posterior teeth restored with amalgam, composite and zirconomer - a stereomicroscopic study. J Clin Diagn Res. 2015;9:ZC65–ZC67. doi: 10.7860/JCDR/2015/13024.6225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El Sayed HY, Abdalla AI, Shalby ME. Marginal microleakage of composite resin restorations bonded by desensitizing one step self etch adhesive. Tanta Dent J. 2014;11:180–188. [Google Scholar]
- 26.Van Meerbeek B, Dhem A, Goret-Nicaise M, Braem M, Lambrechts P, VanHerle G. Comparative SEM and TEM examination of the ultrastructure of the resin-dentin interdiffusion zone. J Dent Res. 1993;72:495–501. doi: 10.1177/00220345930720020501. [DOI] [PubMed] [Google Scholar]
- 27.Wieczkowski G, Jr, Yu XY, Davis EL, Joynt RB. Microleakage in various dentin bonding agent/composite resin systems. Oper Dent. 1992;(Supplement 5):62–67. [PubMed] [Google Scholar]
- 28.Nakabayashi N, Takarada K. Effect of HEMA on bonding to dentin. Dent Mater. 1992;8:125–130. doi: 10.1016/0109-5641(92)90067-m. [DOI] [PubMed] [Google Scholar]
- 29.Vichi A, Margvelashvili M, Goracci C, Papacchini F, Ferrari M. Bonding and sealing ability of a new self-adhering flowable composite resin in class I restorations. Clin Oral Investig. 2013;17:1497–1506. doi: 10.1007/s00784-012-0846-6. [DOI] [PubMed] [Google Scholar]
- 30.Tjäderhane L, Nascimento FD, Breschi L, Mazzoni A, Tersariol IL, Geraldeli S, Tezvergil-Mutluay A, Carrilho M, Carvalho RM, Tay FR, Pashley DH. Strategies to prevent hydrolytic degradation of the hybrid layer - a review. Dent Mater. 2013;29:999–1011. doi: 10.1016/j.dental.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Landuyt KL, Snauwaert J, De Munck J, Peumans M, Yoshida Y, Poitevin A, Coutinho E, Suzuki K, Lambrechts P, Van Meerbeek B. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials. 2007;28:3757–3785. doi: 10.1016/j.biomaterials.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 32.Yoshihara K, Nagaoka N, Hayakawa S, Okihara T, Yoshida Y, Van Meerbeek B. Chemical interaction of glycero-phosphate dimethacrylate (GPDM) with hydroxyapatite and dentin. Dent Mater. 2018;34:1072–1081. doi: 10.1016/j.dental.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Kenshima S, Francci C, Reis A, Loguercio AD, Filho LE. Conditioning effect on dentin, resin tags and hybrid layer of different acidity self-etch adhesives applied to thick and thin smear layer. J Dent. 2006;34:775–783. doi: 10.1016/j.jdent.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Dickens SH, Cho BH. Interpretation of bond failure through conversion and residual solvent measurements and Weibull analyses of flexural and microtensile bond strengths of bonding agents. Dent Mater. 2005;21:354–364. doi: 10.1016/j.dental.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Sundfeld RH, Valentino TA, de Alexandre RS, Briso AL, Sundefeld ML. Hybrid layer thickness and resin tag length of a self-etching adhesive bonded to sound dentin. J Dent. 2005;33:675–681. doi: 10.1016/j.jdent.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 36.Alcantara-Galeana MdCZ, Contreras-Bulnes R, Rodríguez-Vilchis LE, Espinosa-Pesqueira ME, Barrera-Ortega CC, López-Hurtado IM, Fernández-Bobadilla A. Microhardness, structure, and morphology of primary enamel after phosphoric acid, self-etching adhesive, and Er:YAG laser etching. Int J Opt. 2017;2017:7634739 [Google Scholar]
- 37.Wang R, Shi Y, Li T, Pan Y, Cui Y, Xia W. Adhesive interfacial characteristics and the related bonding performance of four self-etching adhesives with different functional monomers applied to dentin. J Dent. 2017;62:72–80. doi: 10.1016/j.jdent.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 38.Spencer P, Wang Y. Adhesive phase separation at the dentin interface under wet bonding conditions. J Biomed Mater Res. 2002;62:447–456. doi: 10.1002/jbm.10364. [DOI] [PubMed] [Google Scholar]
- 39.Gonçalves L, Amaral CM, Poskus LT, Guimarães JG, Silva EM. Degradation of resin composites in a simulated deep cavity. Braz Dent J. 2014;25:532–537. doi: 10.1590/0103-6440201300089. [DOI] [PubMed] [Google Scholar]