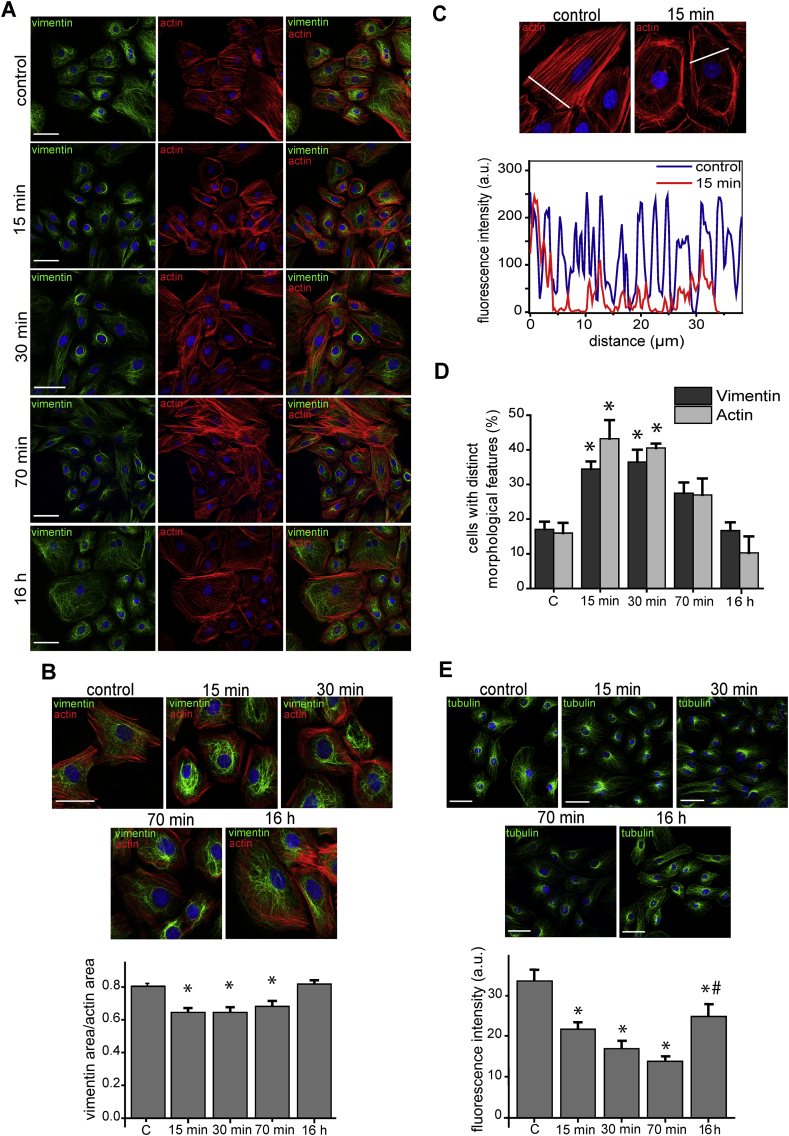
Fig. 1.
Morphological changes of actin, vimentin and tubulin induced by nitroxidative stress. (A) Rat primary cardiac cells were treated with SIN-1 (10 μmol/L) for the indicated times and the distribution of f-actin (red) and vimentin (green), was assessed by phalloidin staining and immunofluorescence, respectively. Nuclei were counter-stained with DAPI (blue) in all panels. (B) The distribution of f-actin and vimentin are shown in more detail. Images are representative of three independent experiments. Scale bars, 50 μm. The cellular areas covered by the vimentin and the actin cytoskeletons were measured by FIJI and their ratios calculated for at least 20 cells per condition. Data are shown in the lower histogram as mean ± SEM (*p <0.005 vs. control, depicted as “C” in the graph). (C) The distribution of f-actin was assessed as in (A) and the fluorescence intensity profiles along the white lines were obtained with FIJI and are depicted in the lower panel as a function of the distance. (D) Proportion of cells showing distinct morphological features, characteristic of nitroxidative stress, as detailed in the text, namely, perinuclear condensation for vimentin and loss of stress fibers for actin. Data are shown as mean ± SEM (*p <0.01 vs. control, “C”) of 40–50 cells per condition, obtained from three independent experiments. (E) Cells were treated with SIN-1, as indicated, and the distribution of β-tubulin was monitored by immunofluorescence. The lower panel represents the density of the microtubule network at the cell periphery estimated from the fluorescent intensity of an area adjacent to cell membrane. Graphed values are mean ± SEM of at least 20 cells per time point (*p <0.05 with respect to control, “C”, #p <0.05 with respect to the 70 min time point); a. u., arbitrary units. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)