Fig. 2.
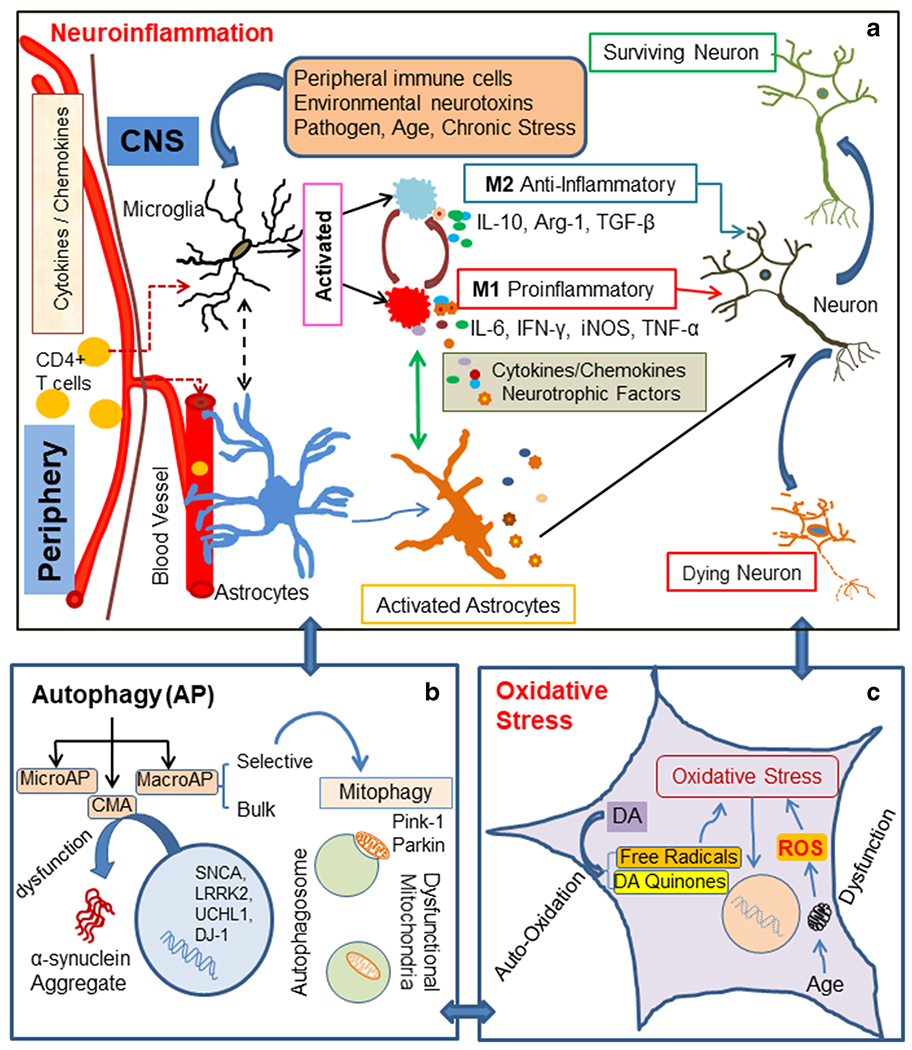
The diagram depicts several pathways involved in PD pathophysiology, a Neuroinflammation – Microglia are resident immune cells activated by various stimuli, e.g., environmental neurotoxins, pathogens, peripheral inflammation (CD4 + T cells), age, and chronic stress. These stimuli may promote divergent microglial phenotypes such as M1, a proinflammatory phenotype, which can be toxic to neurons. M1-type microglia secrete proinflammatory cytokines such as IL-1β, IL-6, IFN-γ, TNF-α, complement proteins, inducible nitric oxide synthase (iNOS), and reactive oxygen species (ROS). M2, an antiinflammatory phenotype microglia, is believed to be neuroprotective. They secrete cytokines and growth factors such as IL-10, TGF-β, brain-derived neurotrophic factor (BDNF), and arginase-1 (Arg-1). Crosstalk between microglia and astrocytes may promote a time dependent secretion of cytokines/growth factors, contributing to PD pathological conditions, b Autophagy - Neuroinflammation influences all three types of autophagy: (i) macroautophagy (MacroAP), (ii) microautophagy (MicroAP), and (iii) chaperon mediated autophagy (CMA). Genetic mutations may contribute to dysfunction of CMA, leading to α-synuclein aggregation. Mitophagy, a selective autophagy process, can clear dysfunctional mitochondria. Pink1 and Parkin maintain mitochondrial cytoarchitecture and function; they also regulate mitophagy. c Oxidative stress - Autooxidation of dopamine (DA) in dopaminergic neurons generates free radicals and DA quinone. These free radicals lead to oxidative stress. Associated with aging and specific toxins, mitochondrial dysfunction causes the generation of ROS, which ultimately results in oxidative cellular damage
