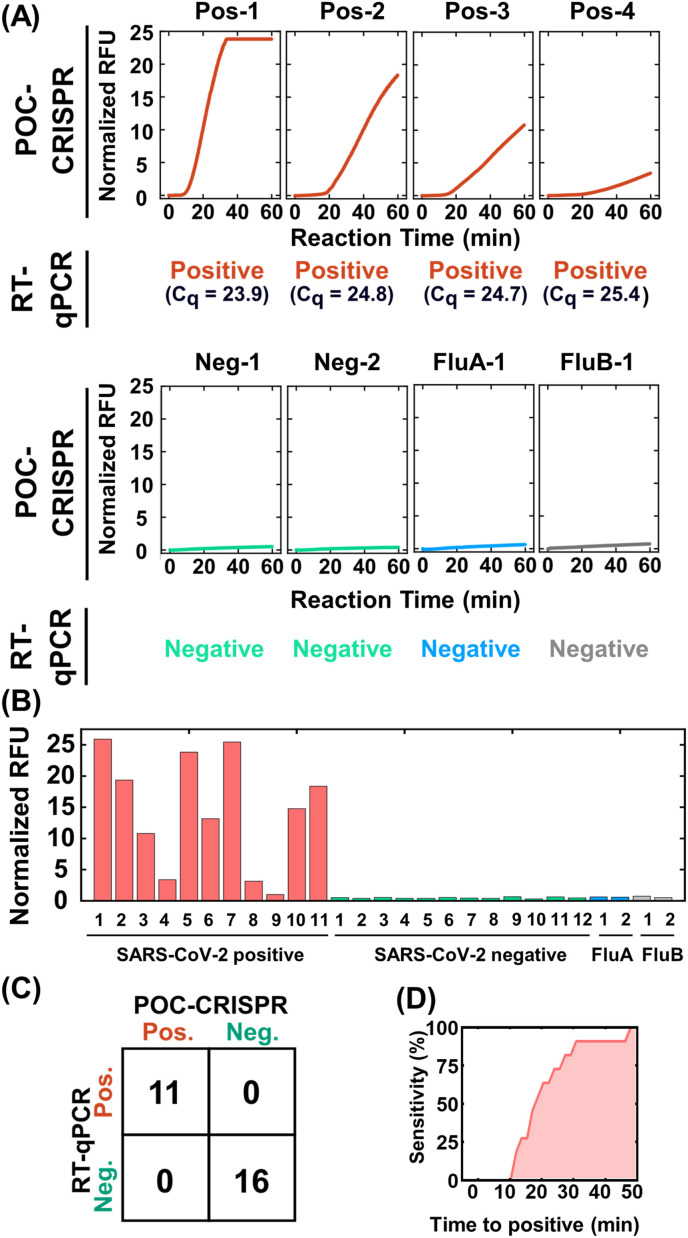
Fig. 4.
Detection of SARS-CoV-2 from unprocessed clinical NP swab eluates via POC-CRISPR. Twenty-seven clinical NP swab eluates that are pre-typed by standard RT-qPCR (11 positive and 16 negative including 2 Inflenza A and 2 Influenza B samples) are tested by POC-CRISPR without prior sample processing steps. (A) SARS-CoV-2 positive samples result in fluorescence amplification curves, whereas negative samples – including the Influenza samples – yield negligible fluorescence increases. (B) By comparing the normalized endpoint (i.e., 60 min) fluorescent intensities after POC-CRISPR, the fluorescent signals of SARS-CoV-2 positive samples are higher than the SARS-CoV-2 negative samples, resulting in (C) 27 out of 27 concordance between POC-CRISPR and benchtop RT-qPCR. (D) By virtue of real-time fluorescence detection, POC-CRISPR accelerates testing turnaround time without sacrificing the sensitivity for detecting the 11 positive samples. Indeed, 7 out of the 11 positive samples are identified in 20 min, and all of the 11 positive samples are detected in 50 min.