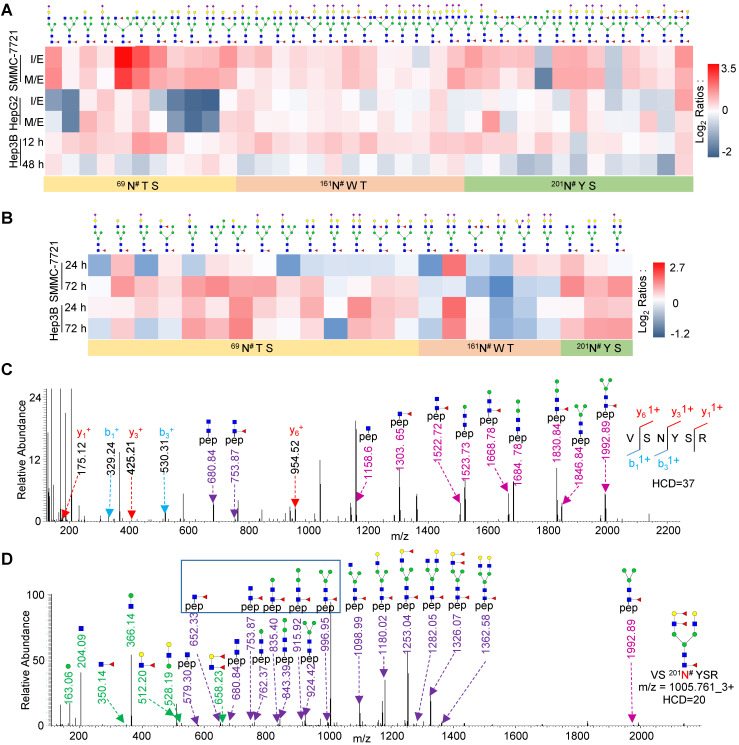
Figure 3.
Site-specific glycan profiling of highly core-fucosylated FOLR1. A. Heat map showing identified glycans at the glycosite Asn-69, Asn-161, Asn-201 of FOLR1 in three cell lines with HGF treatment. B. Heat map showing identified intact glycopeptides from FOLR1 in SMMC-7721 and Hep3B cells with TGF-β1 treatment. C, D. Representative MS/MS spectra for identification of an intact glycopeptide from FOLR1. C. Identification of the peptide sequence VS201N#YSR using a MS/MS spectrum with high energy HCD fragmentation. #indicates the glycosylation site. D. Determination of the glycan structure HexNAc4Hex5Fuc3 attached at the glycosite Asn-201 using a MS/MS spectrum with low energy HCD fragmentation. Core-fucosylation was identified by five feature Y ions (from peptide+HexNAc1Fuc1 ion at m/z=652.332+ to peptide+HexNAc2Hex3Fuc1 ion at m/z=996.952+). The outer arm fucosylation was determined based on the feature B ions (HexNAc1Hex1Fuc2, HexNAc1Hex1Fuc1, and HexNAc1Fuc1 but no HexNAc1Fuc2) as well as the related Y ions. The m/z values of Y ions with charge states 1+ and 2+ are labeled by light and dark purple, respectively.