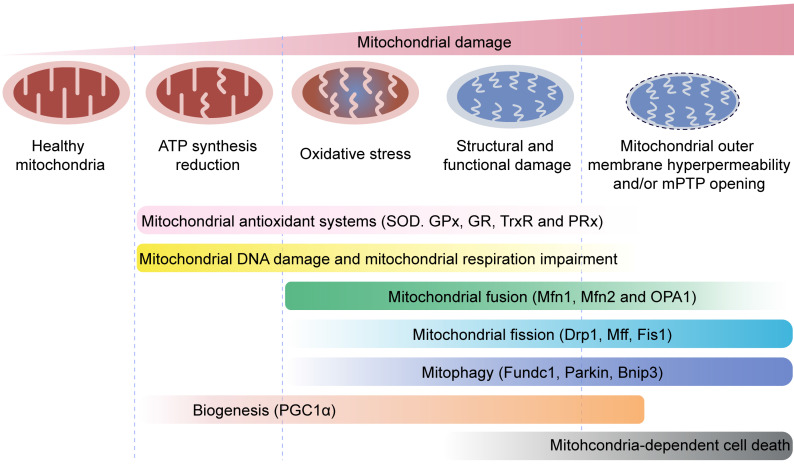
Figure 5.
Mitochondrial quality control mechanisms. Mild mitochondrial stress is accompanied by decreased ATP production and mitochondrial oxidative stress. This stimulates mitochondrial anti-oxidative enzymes such as superoxidase dismutase (SOD), glutathione reductase (GR), glutathione peroxidases (GPXs), thioredoxin reductase (TrxR), and peroxiredoxin (PRx) to reduce the levels of mitochondrial reactive oxygen species (mROS). Mitochondrial injury is followed by mitochondrial DNA damage, which impairs transcription and translation of mitochondria-encoded electron transport chain proteins. Severe mitochondrial stress is characterized by aberrant alterations in the mitochondrial structure and function. This induces mitochondrial fusion mediated by mitofusin 1 (Mfn1), mitofusin 2 (Mfn2), and optic atrophy 1 (OPA1). Mitochondrial fusion allows mixing of damaged and healthy mitochondria to restore functional homeostasis. Meanwhile, mitochondrial fission is activated by cytosolic dynamin-1-like protein (Drp1) and its receptors, and results in segregation of damaged mitochondria that are then targeted for degradation by mitophagy through specific adaptors such as FUN14 domain-containing 1 (Fundc1), Parkin, and BCL-2/adenovirus E1B 19 kDa protein-interacting protein 3 (Bnip3). Excessive mitochondrial damage and stress promotes increased mitochondrial fission that converts reticular mitochondrial network into punctate and fragmented mitochondria. This reduces ATP production and affects cellular growth and survival. Besides, excessive mitophagy significantly reduces mitochondrial mass leading to bioenergetic crisis and cell death or apoptosis. Newer mitochondria are synthesized by mitochondrial biogenesis through specific transcription factors such as peroxisome proliferator-activated receptor-γ co-activator 1α (PGC1α). However, excessive mitochondrial damage induces cell death.