Figure 1. Immune cell involvement after cardiac injury.
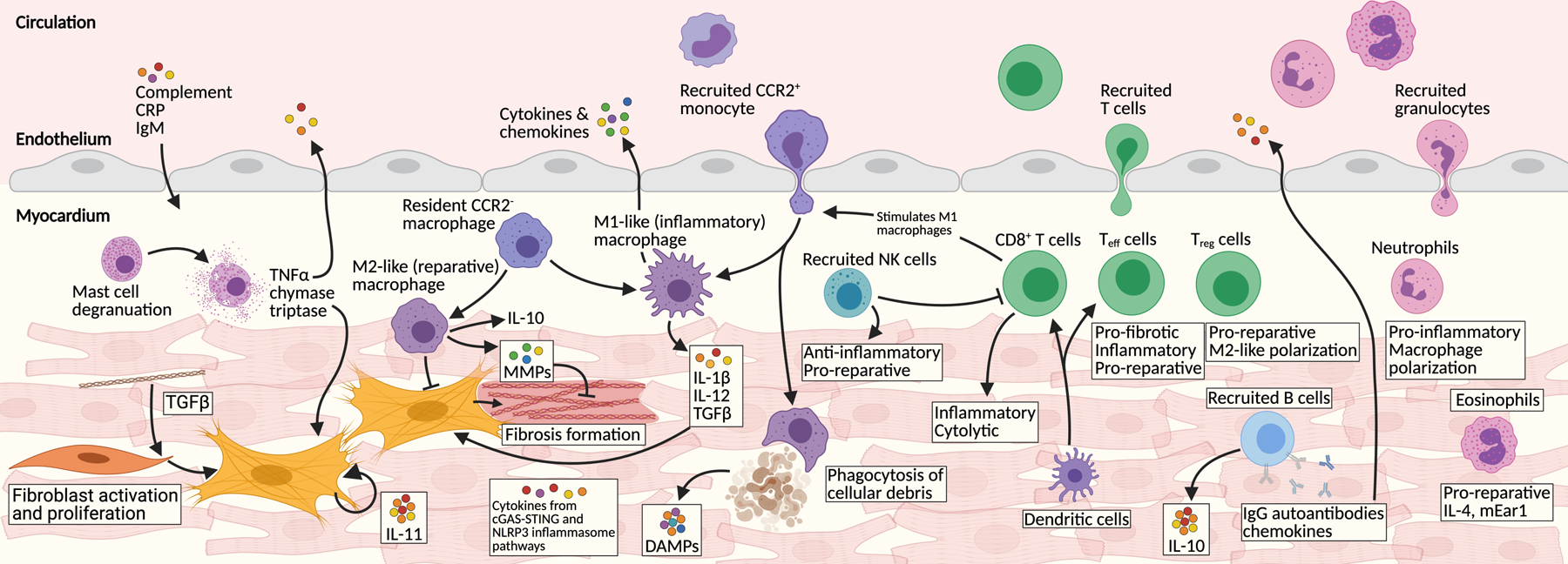
Numerous immune components are involved in the injured myocardium. The main components are represented here. Stressed cells initiate inflammatory secretory programs through the cytosolic DNA sensing cGAS-STING pathway. Dying cells release damage associated patterns (DAMPs) which act as potent signaling molecules. C-reactive protein (CRP), IgM, and other circulating factors permeate the injured heart. Tissue-resident macrophages proliferate and additional CCR2+ monocytes are recruited from circulation. Macrophages polarize between M1-like (inflammatory) and M2-like (reparative) phenotypes. Dendritic cells are potent activators of recruited T cells, which are major immune infiltrates. CD8+ cytotoxic and several effector T (Teff) cells signal with M1 macrophages and many other pro-inflammatory pathways. In contrast some Teff and regulatory T cells (Treg) infiltrate the heart and stimulate M2-like macrophages, ECM remodeling and overall heart repair. B cells produce many chemokines, cytokines, and autoantibodies. Natural killer (NK) cells are recruited and play a mainly reparative role. Many neutrophils are recruited where they stimulate M1-like macrophages and secrete pro-inflammatory cytokines. Eosinophils also infiltrate the heart and help stimulate tissue recovery. One of the major downstream targets of this inflammatory milieu are cardiac fibroblasts. Tissue-resident fibroblasts proliferate and activate and produce detrimental fibrosis.
