Abstract
Understanding photoisomerization dynamics in cyanobacteriochromes is important to development of optical agents in near-infrared biological imaging and optogenetics. Here, integrating femtosecond spectroscopy and site-directed mutagenesis, we investigate photoinduced Pr-state isomerization dynamics and mechanism of a unique red/green cyanobacteriochrome from Leptolyngbya sp. JSC-1. We observed multiphasic dynamics in the Pr state, a widespread phenomenon for photoreceptors in the phytochrome superfamily, and revealed their origins; the initial dynamics in a few to tens and hundreds of picoseconds arises from the local active-site relaxations followed by the slow double-bond isomerization in several hundreds of picoseconds. Such continuous active-site evolution results in a unique spectral tuning effect that favors the blue-side emission and suppresses the red-side emission. We also observed the faster dynamics both in relaxation and isomerization with critical mutants at the active site that render a looser active site. These results clearly distinguish the multiphasic dynamics between relaxation and isomerization and reveal a novel molecular mechanism of what for better biological applications.
Keywords: Active-site relaxation, spectral tuning, double-bond twisting, conical intersection, femtosecond spectroscopy
Graphical Abstract
Genetically encoded fluorescent proteins have been widely used in modern super-resolution fluorescence microscopy to obtain spatial and temporal information of in vivo physiological processes.1 Several cyanobacteriochrome-based near-infrared probes have been successfully developed for noninvasive in vivo imaging.2–4 Cyanobacteriochromes (CBCRs) are bilin-based modular photoreceptors in cyanobacteria that exhibit extremely diverse spectral properties covering from near-ultraviolet to far-red region.5–9 The primary photochemical reaction is the photoinduced isomerization at the C15=C16 double bond in the covalently-linked linear tetrapyrrole chromophore.10 The CBCRs are excellent templates for engineering fluorescence probes due to their compact size, modular architecture and spectral diversity as well as optical properties of the red-light absorbing Pr (15Z) state. Currently, with only a few time-resolved studies of the Pr-state CBCRs available, the primary photochemical mechanism is not well understood.11–14 In particular, the origin of the reported multiphasic decay dynamics and the effect of protein active-site relaxation on ultrafast photoisomerization dynamics remain unresolved. Thus, a better understanding of the ultrafast photochemical mechanism at a molecular level is urgently needed to facilitate the bioengineering of CBCRs for biological applications.
To address these important issues, we systematically studied the excited Pr-state isomerization dynamics of a recently discovered multidomain sensory histidine kinase PPHK (phosphorylation-responsive photosensitive histidine kinase) from cyanobacterium Leptolyngbya sp. JSC-1 (Figure 1).15,16 In PPHK, a phosphorylation-sensing N-terminal receiver (nREC) domain is connected to a light-sensing GAF (cGMP phosphodiesterase/adenyl cyclase/FhlA) domain by a long helical structure commonly in photosensory proteins.17,18 PPHK is photo-convertible between the red-absorbing Pr (15Z) and green-absorbing Pg (15E) states. The crystal structure of the PPHK Pg state shows that the phycocyanobilin (PCB) chromophore incorporated to the GAF domain through a covalently linkage to a cysteine residue (C241) in a shallow active site is readily accessible to the solvent (Figure 1). PCB engages extensive hydrogen-bonding interactions with the protein moiety through its propionate groups and pyrrole nitrogen atoms. It has been reported that the spectral properties of Pr/Pg states can be tuned by substitutions at the Phe residues near the chromophore.15 We have previously reported the significant active-site solvation dynamics in the Pg state of PPHK, showing that the excited-state isomerization is strongly coupled to the local protein solvation dynamics.19 Here, we report our studies of ultrafast Pr-state photoisomerization dynamics of PPHK by combining both femtosecond (fs)-resolved fluorescence and absorption methods. By measuring the fs-resolved fluorescence signals of the chromophore emission, we are able to follow the entire evolution of the Pr-state photoisomerization dynamics in the wild type (WT) and three critical mutants (E210L, F214L and F249L) at the active site (Figs. 1B and S1). By comparing the dynamic properties of WT and mutants, we show that the active-site environment plays a critical role on the Pr-state photoisomerization reaction. These results together reveal a unique dynamic pattern of excited-state evolution and thus elucidate the molecular mechanism of Pr-state photoisomerization in cyanobacteriochrome.
Figure 1.
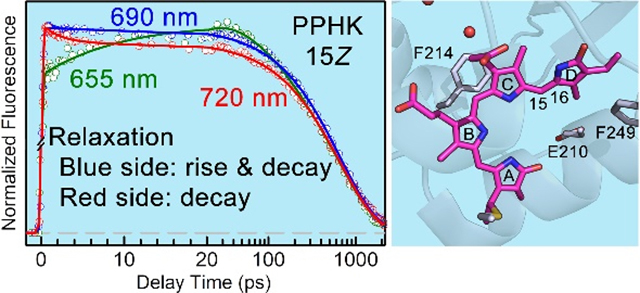
(A) Surface and ribbon representations of Pr-state PPHK dimer. The nREC domain (red) is connected to the GAF domain (blue) through a long helical structure as commonly observed in the phytochrome family. The chromophore (purple) is covalently linked to the GAF domain and exposed to the solvent. (B) Close-up view of the local chromophore binding pocket with water molecules found in the crystal structure (PDB ID: 6OB8). The chromophore is in close contact with the negatively-charged D211 (blue) and E210 (green). The Phe residues of F214 and F249 (green) are also shown.
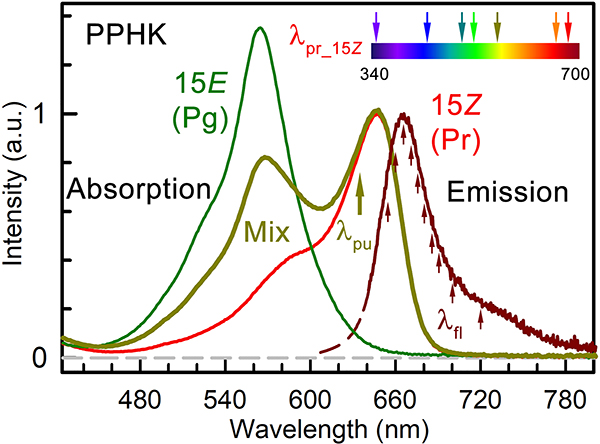
Figure 2 shows the WT steady-state absorption spectra of Pr, Pg and a mix state as well as the fluorescence spectrum of the Pr state. The Pr state is characterized by a peak absorption at 647 nm with a shoulder near 580 nm. The Pr-state fluorescence spectrum is approximately the mirror image of its absorption spectrum with a major peak at 665 nm that extends beyond 800 nm. All three mutants of E210L, F214L, and F249L at the active site show similarity in their overall shapes of both absorption and emission spectra (Figure S2, Table S1). However, the mutants at the conserved Phe residues around the chromophore exhibit small spectral tuning effects. Specifically, the Pr-state absorption peak is blue-shifted by 5 nm in the F214L mutant compared to that of the WT while its fluorescence peak is largely unchanged. Furthermore, both the F214L and F249L mutants show the altered photoconversion efficiency (Figure S2, Table S1), with the increased Pr-state portion in the mix state compared to that of WT (62% Pr state). In contrast, the Pr-state composition is dramatically decreased to around 37% in the E210L mutant although the charged sidechain does not directly interact with the chromophore. Interestingly, the two Pr/Pg ground states in all these proteins are in equilibrium at room temperature.
Figure 2.
Steady-state absorption spectra of the Pg, Pr and mix states of PPHK. The mix state is under constant 530 nm-peak LED illumination. The pure Pr-state spectrum was obtained by conservative subtraction of pure Pg state spectrum from the mix state. The pump wavelength at 635 nm and multiple gated fluorescence wavelengths are marked in arrows. The transient-absorption probe wavelengths are also marked by top arrows.
To characterize the excited Pr-state dynamics, we directly measured the fs-resolved fluorescence dynamics of WT gated from 655 to 720 nm upon 635-nm excitation (Figures 3, S3 and S4). Figure 3 shows the fs-resolved fluorescence transients at several typical wavelengths. In addition to the common component of 640-ps lifetime, we observed a decay component in ~2 ps on the red side of the emission from 690 nm. On the blue side from 655 to 690 nm, we observed a rise component in 12–20 ps followed by a decay component in ~115 ps (Table S2). Such a spectral evolution pattern is unique, similar to our previous observation of Pr-state photoisomerization in bacteriophytochrome (PaBphP),20 and opposite to a usual solvation pattern with decay at the blue side and rise on the red side of the typical Stokes shifts.19,21,22 We constructed the fs-resolved emission spectra (FRES) from above fs-resolved transients (Figure S4) and observed that the transient at 690 nm only exhibits the lifetime decay in 640 ps. The unique evolution must reflect the special relaxation of protein sidechains and local water molecules at the active site upon excitation of the Pr-state chromophore. Such an unusual dynamical pattern indicates the unique spectral tuning on the excited potential surface, which results in fluorescence emission favoring the blue side and disfavoring the red side while the total spectral range remains nearly unchanged. Together with the Pr (15Z)-state in phytochrome PaBphP,20 we found that for the 15Z bilin chromophores share a common feature in spectral evolution; a largely unaltered emission profile is accompanied by subtle but consistent spectral distortions with the rise (and the decay) at the blue side and decay on the red side, and such a tuning effect of the excited potential energy surface arises from the local relaxation of the chromophore-binding pocket. Similar observations of the active-site relaxations in other phytochromes have recently been reported by other biophysical methods.23–25
Figure 3.
Normalized femtosecond-resolved fluorescence transients of WT PPHK from seven selected wavelengths in the Pr state in a short time range (left) and on a long time scale (right). All experimental data are shown in circles, and the solid lines are the best exponential fit.
To fully characterize the Pr excited-state isomerization and possible intermediates, we switched to the transient-absorption detection as shown in Figure 4. Specifically, we detected a ground-state recovery at 350 nm in hundreds of picoseconds. The observed negative plateau at long time is attributed to the formation of the photoproduct Lumi-R. Transient signals probed at 440, 500 and 520 nm mainly reflect the excited-state dynamics and active-site relaxations on picosecond timescales, which are consistent with the fluorescence experiments.19, 21, 22, 26, 27 At 560 nm, the excited-state absorption and ground-state bleaching signals are nearly cancelled out while the observed dynamics in tens to hundreds of picoseconds are resulted from the active-site relaxation including protein sidechains, trapped water molecules and hydrogen-bond networks. Transient signals probed at 660 and 680 nm are negative, due to stimulated emission and product formation with minimum contributions from ground-state bleaching signals. The WT transients are fitted with sum of various exponential decays and the fitting results are listed in Table S3. Besides the dominant excited-state lifetime component, we observed three components with time constants of about 1.2–3.8, 17 and 131–150 ps associated with the active-site relaxations. These ultrafast transients are in good agreement with those obtained by fs-resolved fluorescence dynamics. Taken together, these data support that the multiphasic decays mainly arise from the active-site relaxations, rather than ground- or excited-state heterogeneity.28
Figure 4.
Normalized femtosecond-resolved absorption transients of WT PPHK probed from 350 to 680 nm. (A) The gradual changes of the dynamics with different probe wavelengths. (B-C) the deconvolution of the transients into various dynamic components probed at 560 nm on short and long time scales. All the experimental data are shown in circles and the solid lines are the best exponential fit. The dashed lines are deconvoluted components.
To further examine the effect of the protein-chromophore interactions in the active site on the primary photochemical reaction dynamics in phytochrome and cyanobacteriochromes,19,20,29 we mutated several critical active-site residues one at time. Figure 5 shows the transients of three mutants of E210L, F214L and F249L with WT as comparison for two typical probing wavelengths at 500 nm and 680 nm out of a series of probed wavelengths. The active-site relaxation is clearly observed although the excited-state lifetimes vary among mutants (Figures S5–S7, Tables S4–S6). F249L exhibits the excited-state lifetime component of 480 ps with active-site relaxation dynamics ranging from 1.5 to 86 ps. The bulky sidechain of F249 in the vicinity of the D-ring may exert steric hindrances to the D-ring motions upon photoisomerization, as a rotating D-ring pushes away the aromatic ring of F249 in a diffusive manner.30 Indeed, we observed the accelerated excited-state dynamics in the F249L mutant. The F214L mutant also exhibits similar accelerated photoisomerization dynamics in 540 ps while the active-site relaxation occurs in 1.2 to 135 ps. The aromatic sidechain of F214 is parallel to the A-C ring plane, potentially forming π-π stacking interactions with PCB. The absence of a bulky sidechain near the chromophore would accelerate photoisomerization followed by structural rearrangements in PCB and protein moiety in the later stages of the photoconversion.30–33 Overall, the Phe mutations of F214L and F249L give rise to a flexible active site, which allows faster relaxation and isomerization dynamics. In contrast, the E210L mutant does not seem to affect the Pr-state dynamics with a 630-ps component and relaxation dynamics from 1.4 to 190 ps similar to WT. E210 is located right next to D211, a highly conserved residue that forms direct hydrogen-bonding interactions with the pyrrole nitrogen atoms in the A- to C- rings of PCB (Figures 1B and S1).16 It is noteworthy that the E210L mutation affects the Pg-state dynamics with faster solvation dynamics and excited-state lifetime compared to WT.19 It is highly plausible that such distinct photophysical properties of PCB are due to the different protein-chromophore interactions in the Pr and Pg states.19
Figure 5.
Normalized femtosecond-resolved absorption transients of WT and mutants probed at 500 nm (A) and 680 nm (B). Insets show the absorption transients at early delay times. (C-E) The deconvolution of transients probed at 500 nm of E210L and F249L, and at 680 nm of F214L into multi-exponential components.
In summary, we reported our systematic characterization of the Pr-state photoisomerization dynamics of a cyanobacteriochrome PPHK. Using fs-resolved fluorescence and absorption spectroscopy, we are able to distinguish the active-site relaxation and isomerization dynamics in PPHK. The active-site relaxation takes place in a few to tens and hundreds of picoseconds, revealing a continuous structural evolution upon photoexcitation of the chromophore and resulting in a unique spectral tuning effect on the emission spectra (Figure 6A). The isomerization, a barrier-crossing process, occurs in hundreds of picoseconds after the active-site relaxation. Together, these two different processes, namely faster active-site relaxation and slower double-bond isomerization, give rise to the multiphasic ultrafast dynamics in the Pr state before the photoreceptor passes through a conical intersection to reach the ground-state intermediates (Figure 6B). With three critical mutants (F214L, F249L, E210L) in the close vicinity of the chromophore, we have shown that the protein active site plays a critical role on the Pr-state dynamics, with faster relaxation and isomerization in a flexible pocket. Overall, the findings of this work have revealed the origins of the multiphasic dynamics commonly observed in phytochrome systems. The observed spectral tuning effect on the excited potential surface also provides a different roadmap for engineering near-infrared fluorescent proteins based on phytochrome photoreceptors. An important message of this work is that one must consider the active-site relaxation in interpreting multiphasic dynamics in other CBCRs and phytochromes.
Figure 6.
(A) Dissection of the fluorescence emission corresponding to the different evolution at the blue and red side. The corresponding transients are shown on the top. (B) Schematic potential energy surface for PPHK Pr-state photoisomerization along the reaction coordinate. The structural relaxation is in a few to hundreds of picoseconds and the excited-state isomerization in 640 ps. The excited-state population decays to the ground state through the conical intersection (CI).
Supplementary Material
ACKNOWLEDGMENTS
We also thank Dr. Sheng Zhang for the initial help in experiment. This work was supported in part by the National Institute of Health grants GM118332 to DZ and EY024363 to XY.
Footnotes
The authors declare no competing financial interests.
ASSOCIATED CONTENT
The following files are available free of charge.
Materials and methods, supporting figures and tables, and additional references. (PDF)
REFERENCES
- 1.Shcherbakova DM; Shemetov AA; Kaberniuk AA; Verkhusha VV Natural Photoreceptors as a Source of Fluorescent Proteins, Biosensors, and Optogenetic Tools. Annu. Rev. Biochem 2015, 84, 519–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oliinyk OS; Chernov KG; Verkhusha VV Bacterial Phytochromes, Cyanobacteriochromes and Allophycocyanins as a Source of Near-Infrared Fluorescent Probes. Int. J. of Mol. Sci 2017, 18, 1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shcherbakova DM; Stepanenko OV; Turoverov KK; Verkhusha VV Near-Infrared Fluorescent Proteins: Multiplexing and Optogenetics across Scales. Trends Biotechnol. 2018, 36, 1230–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oliinyk OS; Shemetov AA; Pletnev S; Shcherbakova DM; Verkhusha VV Smallest Near-Infrared Fluorescent Protein Evolved from Cyanobacteriochrome as Versatile Tag for Spectral Multiplexing. Nat. Commun 2019, 10, 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirose Y; Shimada T; Narikawa R; Katayama M; Ikeuchi M Cyanobacteriochrome CcaS is the Green Light Receptor that Induces the Expression of Phycobilisome Linker Protein. Proc. Natl. Acad. Sci. U. S. A 2008, 105, 9528–9533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rockwell NC; Martin SS; Feoktistova K; Lagarias JC Diverse Two-Cysteine Photocycles in Phytochromes and Cyanobacteriochromes. Proc. Natl. Acad. Sci. U. S. A 2011, 108, 11854–11859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Narikawa R; Enomoto G; Ni-Ni-Win; Fushimi K; Ikeuchi M A New Type of Dual-Cys Cyanobacteriochrome GAF Domain Found in Cyanobacterium Acaryochloris marina, Which Has an Unusual Red/Blue Reversible Photoconversion Cycle. Biochemistry 2014, 53, 5051–5059. [DOI] [PubMed] [Google Scholar]
- 8.Rockwell NC; Martin SS; Lagarias JC Identification of DXCF Cyanobacteriochrome Lineages with Predictable Photocycles. Photochem. Photobiol. Sci 2015, 14, 929–941. [DOI] [PubMed] [Google Scholar]
- 9.Rockwell NC; Duanmu D; Martin SS; Bachy C; Price DC; Bhattacharya D; Worden AZ; Lagarias JC Eukaryotic Algal Phytochromes Span the Visible Spectrum. Proc. Natl. Acad. Sci. U. S. A 2014, 111, 3871–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anders K; Essen L The Family of Phytochrome-like Photoreceptors: Diverse, Complex and Multi-colored, but very Useful. Curr. Opin. Struct. Biol 2015, 35, 7–16. [DOI] [PubMed] [Google Scholar]
- 11.Xu X; Gutt A; Mechelke J; Raffelberg S; Tang K; Miao D; Valle L; Borsarelli CD; Zhao K; Gärtner W Combined Mutagenesis and Kinetics Characterization of the Bilin-Binding GAF Domain of the Protein Slr1393 from the Cyanobacterium Synechocystis PCC6803. Chembiochem 2014, 15, 1190–1199. [DOI] [PubMed] [Google Scholar]
- 12.Kim PW; Freer LH; Rockwell NC; Martin SS; Lagarias JC; Larsen DS Femtosecond Photodynamics of the Red/Green Cyanobacteriochrome NpR6012g4 from Nostoc punctiforme. 2. Reverse Dynamics. Biochemistry 2012, 51, 619–630. [DOI] [PubMed] [Google Scholar]
- 13.Gottlieb SM; Kim PW; Chang C; Hanke SJ; Hayer RJ; Rockwell NC; Martin SS; Lagarias JC; Larsen DS Conservation and Diversity in the Primary forward Photodynamics of Red/Green Cyanobacteriochromes. Biochemistry 2015, 54, 1028–1042. [DOI] [PubMed] [Google Scholar]
- 14.Slavov C; Xu X; Zhao K; Gärtner W; Wachtveitl J Detailed Insight into the Ultrafast Photoconversion of the Cyanobacteriochrome Slr1393 from Synechocystis sp. Biochim. Biophys. Acta, Bioenerg 2015, 1847, 1335–1344. [DOI] [PubMed] [Google Scholar]
- 15.Rockwell NC; Martin SS; Gan F; Bryant DA; Lagarias JC NpR3784 is the Prototype for a Distinctive Group of Red/Green Cyanobacteriochromes using Alternative Phe Residues for Photoproduct Tuning. Photochem. Photobiol. Sci 2015, 14, 258–269. [DOI] [PubMed] [Google Scholar]
- 16.Shin H; Ren Z; Zeng X; Bandara S; Yang X Structural Basis of Molecular Logic OR in a Dual-sensor Histidine Kinase. Proc. Natl. Acad. Sci. U. S. A 2019, 116, 19973–19982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takala H; Bjorling A; Berntsson O; Lehtivuori H; Niebling S; Hoernke M; Kosheleva I; Henning R; Menzel A; Ihalainen JA; Westenhoff S Signal Amplification and Transduction in Phytochrome Photosensors. Nature 2014, 509, 245–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao R; Stock AM Biological Insights from Structures of Two-Component Proteins. Annu. Rev. Microbiol 2009, 63, 133–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang D; Li X; Zhang S; Wang L; Yang X; Zhong D Revealing the Origin of Multiphasic Dynamic Behaviors in Cyanobacteriochrome. Proc. Natl. Acad. Sci. U. S. A 2020, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang D; Qin Y; Zhang M; Li X; Wang L, Yang X; Zhong D The Origin of Ultrafast Multiphasic Dynamics in Photoisomerization of Bacteriophytochrome. J. Phys. Chem. Lett 2020, 11, 5913–5919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nandi N; Bhattacharyya K; Bagchi B Dielectric Relaxation and Solvation Dynamics of Water in Complex Chemical and Biological Systems. Chem. Rev 2000, 100, 2013–2046. [DOI] [PubMed] [Google Scholar]
- 22.Zhang L; Yang Y; Kao Y-T; Wang L; Zhong D Protein Hydration Dynamics and Molecular Mechanism of Coupled Water-Protein Fluctuations. J. Am. Chem. Soc 2009, 131, 10677–10691. [DOI] [PubMed] [Google Scholar]
- 23.Claesson E; Wahlgren WY; Takala H; Pandey S; Castillon L; Kuznetsova Y; Henry L; Panman M; Carrillo M; Kübel J; et al. The Primary Structural Photoresponse of Phytochrome Proteins Captured by a Femtosecond X-Ray Laser. eLife 2020, 9, e53514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slavov C; Fischer T; Barnoy A; Shin H; Rao AG; Wiebeler C; Zeng X; Sun Y; Xu Q; Gutt A; Zhao K-H; Gärtner W; Yang X; Schapiro I; Wachtveitl J The Interplay between Chromophore and Protein Determines the Extended Excited State Dynamics in a Single-Domain Phytochrome. Proc. Natl. Acad. Sci. U.S.A 2020, 117, 16356–16362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fischer T; Xu Q; Zhao K; Gärtner W; Slavov C; Wachtveitl J Effect of the PHY Domain on the Photoisomerization Step of the Forward Pr → Pfr Conversion of a Knotless Phytochrome. Chem. Eur. J 10.1002/chem.202003138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen C; Liu W; Baranov MS; Baleeva NS; Yampolsky IV; Zhu L; Wang Y; Shamir A; Solntsev KM; Fang C Unveiling Structural Motions of a Highly Fluorescent Superphotoacid by Locking and Fluorinating the GFP Chromophore in Solution. J. Phys. Chem. Lett 2017, 8, 5921–5928. [DOI] [PubMed] [Google Scholar]
- 27.Espagne A; Changenet-Barret P; Plaza P; Martin M Solvent Effect on the Excited-state Dynamics of Analogues of the Photoactive Yellow Protein Chromophore. J. Phys. Chem. A 2006, 110, 3393–3404. [DOI] [PubMed] [Google Scholar]
- 28.Gottlieb SM; Kim PW; Rockwell NC; Hirose Y; Ikeuchi M; Lagarias JC; Larsen DS Primary Photodynamics of the Green/Red-absorbing Photoswitching Regulator of the Chromatic Adaptation E Domain from Fremyella diplosiphon. Biochemistry 2013, 52, 8198–8208. [DOI] [PubMed] [Google Scholar]
- 29.Wang D; Qin Y; Zhang S; Wang L; Yang X; Zhong D Elucidating the Molecular Mechanism of Ultrafast Pfr-State Photoisomerization in Bathy Bacteriophytochrome PaBphP. J. Phys. Chem. Lett 2019, 10, 6197–6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y; Linke M; von Haimberger T; Hahn J; Matute R; Gonzalez L; Schmieder P; Heyne K Real-time Tracking of Phytochrome’s Orientational Changes during Pr Photoisomerization. J. Am. Chem. Soc 2012, 134, 1408–1411. [DOI] [PubMed] [Google Scholar]
- 31.Yang X; Ren Z; Kuk J; Moffat K Temperature-Scan Cryocrystallography Reveals Reaction Intermediates in Bacteriophytochrome. Nature 2011, 479, 428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmidt A; Sauthof L; Szczepek M; Lopez MF; Velazquez Escobar F; Qureshi BM; Michael N; Buhrke D; Stevens T; Kwiatkowski D; von Stetten D; Mroginski MA; Krauß N; Lamparter T; Hildebrandt P; Scheerer P Structural Snapshot of a Bacterial Phytochrome in its Functional Intermediate State. Nat. Commun 2018, 9, 4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanchez JC; Carrillo M; Pandey S; Noda M; Aldama L; Feliz D; Claesson E; Wahlgren WY; Tracy G; Duong P; Nugent AC; Field A; Šrajer V; Kupitz C; Iwata S; Nango E; Tanaka R; Tanaka T; Fangjia L; Tono K; Owada S; Westenhoff S; Schmidt M; Stojković EA; Kennis JTM High-Resolution Crystal Structures of Amyxobacterial Phytochrome at Cryo and Room Temperatures. Struct. Dyn 2019, 6, 054701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.