Table 2.
Optimization studies.a
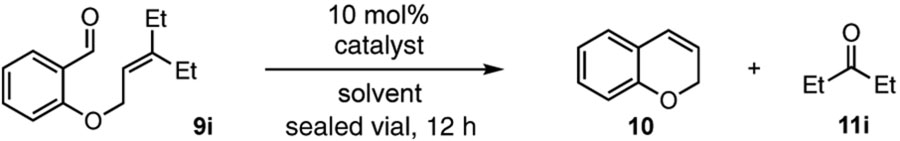 | |||||
|---|---|---|---|---|---|
| entry | catalyst | solvent | temp. (°C) | conv. (%) | 3 yield (%) |
| 1 | 8• (TFA)2 | CH3CN | 140 | 65 | 48 |
| 2 | 8• (TFA)2 | DCE | 140 | 49 | 20 |
| 3 | 8• (TFA)2 | MeOH | 140 | 100 | 40 |
| 4 | 8• (TFA)2 | EtOH | 140 | 100 | 78 (73)b |
| 5 | 8• (TFA)2 | EtOH | 120 | 85 | 47 |
| 6c | TFA | EtOH | 140 | 100 | 0 |
| 7 | FeCl3 | DCE | rt | 100 | 0 |
Conditions: substrate 9i (0.2 mmol) and 10 mol % catalyst in 1.0 mL of solvent in a 5 mL sealed tube were heated to the temperature indicated for 12 h. Conversions and yields were determined by 1H NMR using CH2Br2 as an internal standard.
Isolated yield;
20 mol % TFA was used.
