Fig. 1. Structure of DRM2 in complex with an 18-mer CTT DNA.
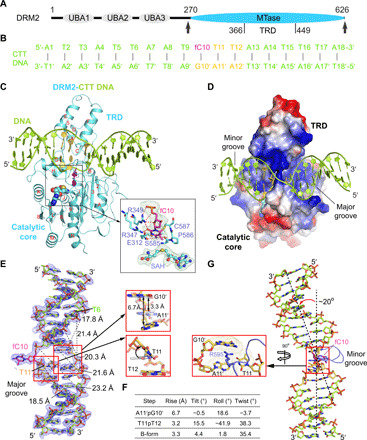
(A) Domain architecture of DRM2, with the methyltransferase (MTase) domain that harbors a target recognition domain (TRD) marked with arrowheads. UBA, ubiquitin-associated domain. (B) The sequence of CTT DNA used for the structural study. fC, 5-fluorocytosine. (C and D) Ribbon (C) and electrostatic surface (D) representations of DRM2 bound to DNA and SAH. DRM2 and bound DNA are colored in aquamarine and limon, respectively. The CTT motif is colored in yellow or purple (fC10). The SAH molecule is shown in sphere representation. The active site is shown in expanded view, with the Fobs-Fcalc omit map (cyan) of fC10 and SAH contoured at 2.0 σ level and hydrogen-bonding interactions depicted as dashed lines. The α helices and β strands are counted in alphabetic and numeric orders, respectively, in (C). The color scheme in (C) is applied to subsequent figures, unless otherwise indicated. (E) The Fobs-Fcalc omit map (blue) of the CTT DNA, contoured at 2.0 σ level. The major groove widths of the deformed DNA upon binding of DRM2 are indicated by dashed lines. Structural alignments of the A11′pG10′ and T11pT12 steps with B-form DNA (gray) are shown in expanded views. (F) Geometric parameters for the DNA base steps boxed in (E). (G) Kinked conformation of DRM2-bound DNA, with the R595 intercalation shown in expanded view.
