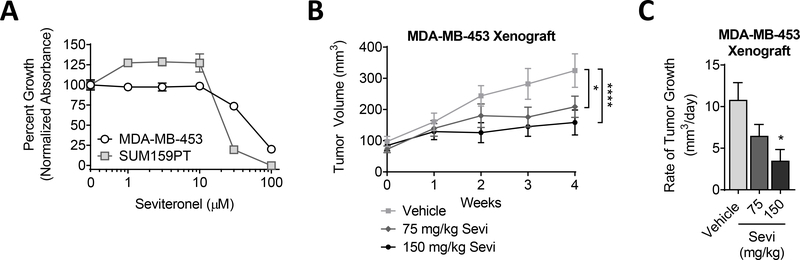
Figure 1. TNBC sensitivity to Sevi in a preclinical models.
(A) TNBC cell lines were cultured in full serum media. Cell viability was determined by crystal violet assay after a 5-day exposure to DMSO (0) or increasing concentrations of Sevi. Data was normalized to the mean absorbance of DMSO-treated cells. Mean ± standard deviation. (B) MDA-MB-453 cells were injected into the mammary fat pads of cycling female nu/nu mice. Supplemental DHT (dihydrotestosterone) was not given in this experiment. Sevi was administered daily. Tumor volume was measured by calipers and (C) the rate of tumor growth per day over four weeks was calculated. Mean ± standard error of the mean; * p<0.05, **** p<0.0001.