Fig. 5. IKKε v2 processes high phosphorylation status through K63-linked ubiquitination.
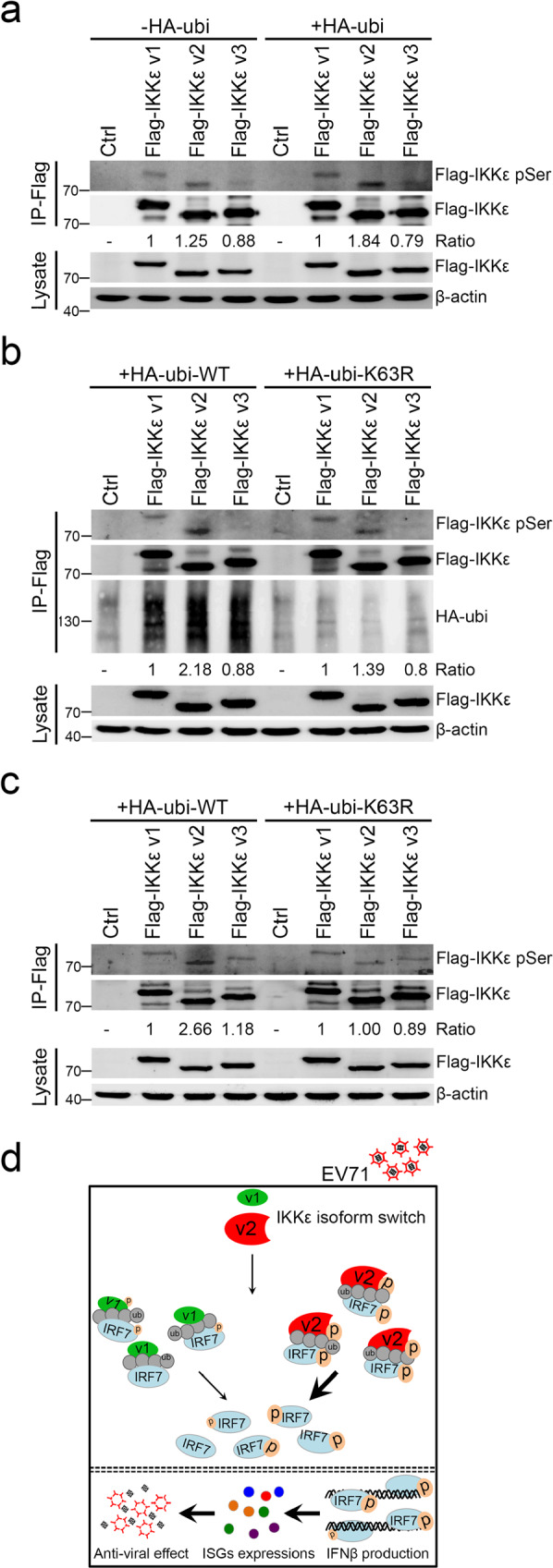
a IKKε v2 presents higher phosphorylation status. HEK293 cells were transfected with each Flag-IKKε isoform accompanied with or without HA-ubi. Immunoprecipitation was performed with anti-Flag beads and the resulting products were detected by anti-phospho-Serine (pSer) and anti-Flag antibodies. b, c K63R ubiquitin markedly reduces IKKε v2 phosphorylation. HEK293 cells were transfected with each Flag-IKKε isoform accompanied with wild-type or K63R HA-ubi. Immunoprecipitation was performed with anti-Flag beads and the resulting products were further assayed by immunoblot with anti-phospho-Serine, anti-Flag, and anti-HA antibodies (b) and in vitro kinase assay (c). β-actin was served as an internal control. d Molecular mechanism of IKKε isoform switching in EV71 infection.
