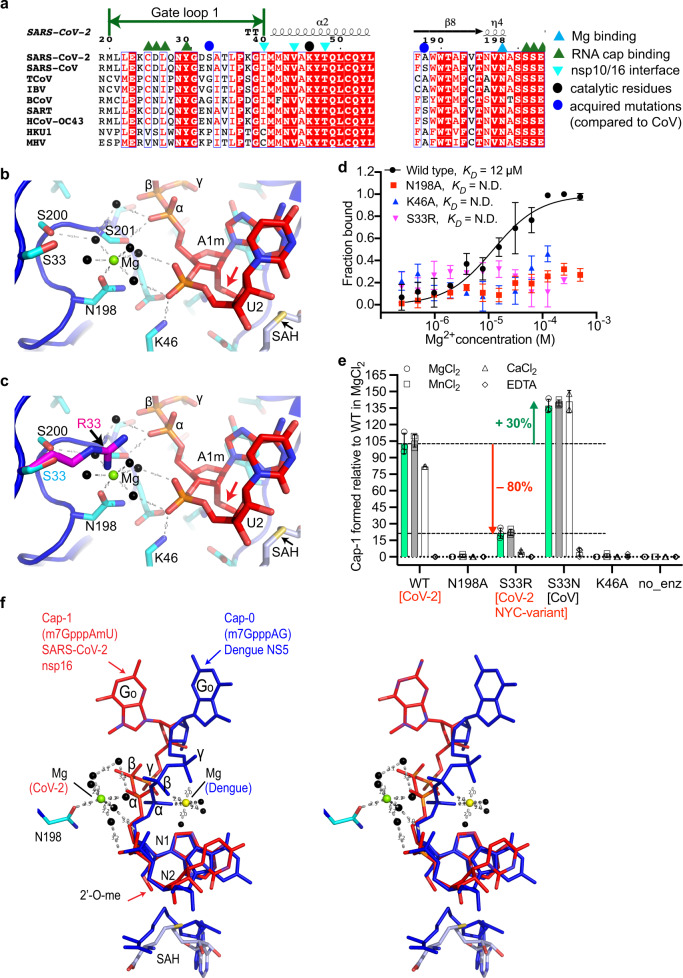
Fig. 2. Metal dependency of nsp16/nsp10 and its clinical variant for 2’-O methylation.
a Alignment of nsp16 from different CoV representing the α, β, γ sub-classes. Blue sphere denotes S33R, which locates in gate loop 1; S33 is an asparagine (N) in SARS-CoV; S33R is a clinical nsp16 variant of SARS-CoV-2; cyan triangle, N198 that coordinates Mg2+; and black sphere, catalytic lysine (K46). b A Mg2+ ion (green sphere) coordinates with five water molecules (black spheres) at the nucleic acid face and side chain of N198 on the opposite face. Two water molecules hold the phosphoryl oxygens of U2. Red arrow; 2’-O-methylated ribosyl of A1 base. c Side chain of arginine (magenta, modeled) at the S33 (cyan) position intrudes into the Mg2+ pocket and may displace Mg2+ or disrupt the Mg2+/water network. d Binding of Mg2+ to nsp16/nsp10 as derived from three independent MST experiments (n = 3) with 1 SD shown as error bars (N.D., not determined). e Quantitative measurement of Cap-1 formation by nsp16/nsp10 enzymes (±metals, ethylenediaminetetraacetic acid [EDTA]) derived from LC/MS. Error bars indicate range of data points from three independent experiments (n = 3, except n = 2 for WT and S33N in CaCl2) normalized to WT in MgCl2. Center of error bars is 102.8%, 105.4%, and 81.9% for WT in MgCl2, MnCl2, CaCl2; 21.4%, 22.4%, and 4.3% for S33R; and 137.1%, 139.6%, and 140.8% for S33N, respectively. N198A, K46A, and EDTA reactions show negligible Cap-1 formation. Spheres and green bars, MgCl2; squares and gray bars, MnCl2; triangles and white bars, CaCl2; and diamonds, EDTA. Source data are provided as a Source Data File. no_enz, reaction devoid of nsp16/nsp10 enzyme. f A stereo view of an overlay of the N1 and N2 bases, and SAH of SARS-CoV-2 nsp16/nsp10 (red) and dengue NS5 (blue; PDB ID: 5DTO). RNA caps show entirely different orientations of the terminal base of the cap (me7G), two phosphates (β and γ) and Mg2+ ions. Mg2+ (in dengue, yellow sphere) stabilizes the three phosphates, whereas in SARS-CoV-2 (green sphere) it indirectly (water-mediated) stabilizes the phosphate of N2 on one side and engages N198 of nsp16 on the opposite side.