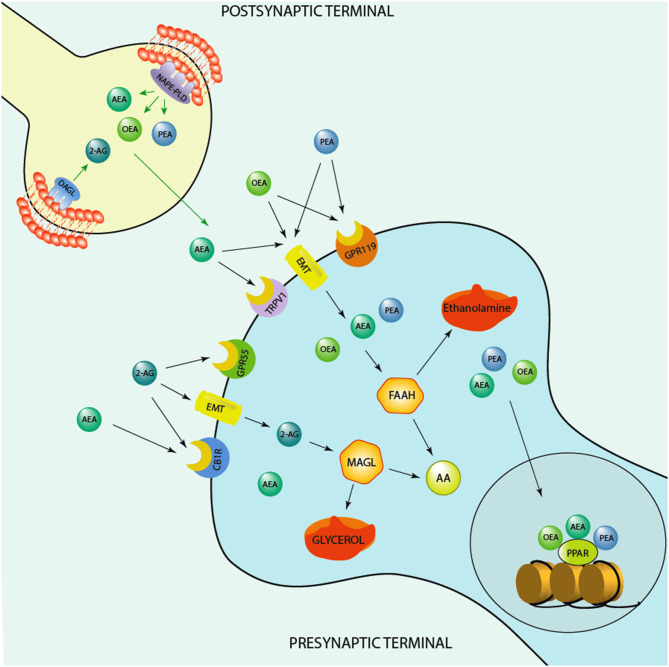
Figure 1.
Schematic representation of the endocannabinoid system. Depicted are several biosynthetic and degradation pathways as well as endocannabinoid receptors that are involved in the action of the endocannabinoids, anandamide (AEA), 2-Arachidonoylglycerol (2-AG), and of the endocannabinoid-like ethanolamines, oleoylethanolamide (OEA) and N-palmitoylethanolamine (PEA). AEA, PEA, and OEA share the similar biosynthetic pathway after originating from membrane's phospholipids are synthesized post-synaptically by the action of the enzyme, N-acylphosphatidylethanolamine-specific phospholipase D (NAPE-PLD). 2-AG is instead produced by the action of the enzyme, diacylglycerol lipase (DAGL), prior to be secreted by post-synaptic terminals and act at pre-synaptic cannabinoid receptor type 1 (CB1) and G protein-coupled receptor 55 (GPR55). AEA, PEA, and OEA can act at membrane receptors or be taken up pre-synaptically through endocannabinoid membrane transporters (EMT). They can be degraded by the action of the enzyme, fatty acid amide hydrolase (FAAH) into ethanolamine and arachidonic acid (AA) pre-synaptically. These endocannabinoids influence each concentration by competing for the catalytic action of FAAH. For instance, increased levels of AEA can compete for the catalytic action of FAAH and thereby result in an increase of PEA and OEA levels or vice versa, PEA and, mostly, OEA by competing for FAAH catalytic action may increase AEA levels. PEA may also decrease FAAH expression and thereby elevate its own and the levels of OEA and AEA. 2-AG is instead degraded by monoacylglycerol lipase (MAGL) to glycerol and AA. While OEA and PEA fail to bind to the classic CB1 and CB2, they can influence the action of AEA at transient receptor potential channels of vanilloid type-1 (TRPV1). PEA may activate peroxisome proliferator-activated receptor-alpha (PPAR-α) as well as TPRV1. What makes the endocannabinoid system attractive for developing novel biomarkers concerns the fact that it is constituted by several components, including synthesizing and degrading enzymes to receptors and endogenous modulators and it is widely distributed in the brain. These neuromodulators are implicated in several mechanisms that regulate neuronal functions, including cognition and emotional behavioral regulation. Likewise, synthetic agents that stimulate endocannabinoid receptors or act on the degrading/biosynthetic enzyme constitute a valid pharmacological approach for treatment of several neuropsychiatric disorders. For instance, the action of AEA binding at CB1 and of PEA at PPAR-α has been associated with a fast improvement of emotional behavioral deficits, including aggressive behavior and impulsivity (11, 12), which are behavioral endophenotypes of human behavioral-traits of suicide risk. In humans, studies show higher CB1 and CB1-mediated G-protein activation in the dorsolateral prefrontal cortex (DLPFC) of suicide victims (13). Studies conducted in alcoholic suicide victims have evidenced enhanced CB1 activation and increased AEA and 2-AG concentrations in the DLPFC (14). Furthermore, CB1 expression was increased in the ventral striatum of suicide individuals who struggled with alcoholism (15). Intriguingly, both FAAH expression and activity was found upregulated in post-mortem brain of suicide subjects (15). Together, these findings underlie profound deficits within the endocannabinoid system. More studies are warranted to understand the precise role of endocannabinoid levels, their biosynthetic enzymes as well as their receptors (CB1 and PPAR-α) in suicide victims.