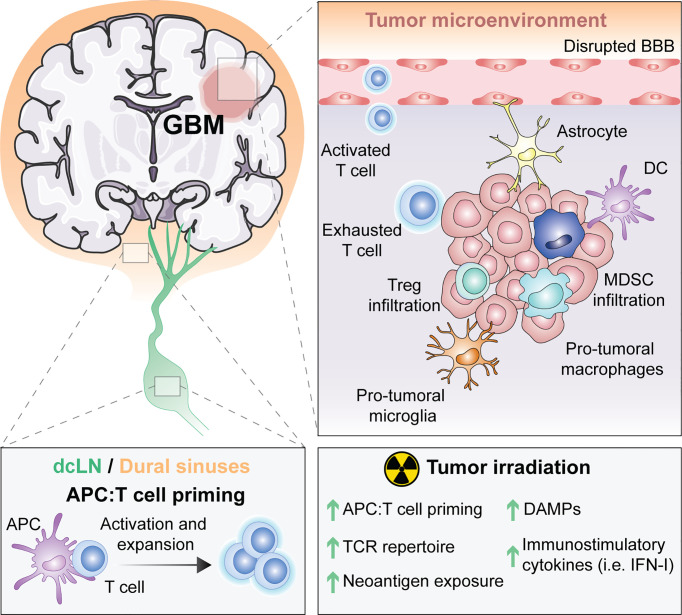
Figure 1.
The unique immune response in GBM and its modulation by RT. For many years, the central nervous system (CNS) was thought to be excluded from immune surveillance. However, it is now known that the CNS is not isolated from activated T cells and that CNS antigens can be presented locally or peripherally in the draining cervical lymph nodes or the dural sinuses. Diverse types of antigen presenting cells (APCs) exist within glioblastoma (GBM), including microglia, macrophages, astrocytes and classic APCs such as dendritic cells (DCs). APCs that have captured tumor antigens can present to naïve T cells, leading to their activation and expansion. Activated T cells migrate into the brain through a disrupted blood brain barrier (BBB), but once in the tumor microenvironment (TME) they differentiate into exhausted T cells. Within the TME, there are immunosuppressive regulatory T cells (Tregs), myeloid derived suppressor cells (MDSC), reactive astrocytes and pro-tumoral macrophages and microglia. Radiotherapy (RT), the standard of care for GBM, induces the exposure of tumor neoantigens and increases the T cell receptor (TCR) repertoire. Moreover, tumor irradiation promotes the release of danger associated molecular patterns (DAMPs) and type I interferon (IFN-I), which stimulate APCs cross-priming of T cells. All of these suggest that RT can be used to overcome GBM immunosuppression to optimally prime anti-tumor immunity.