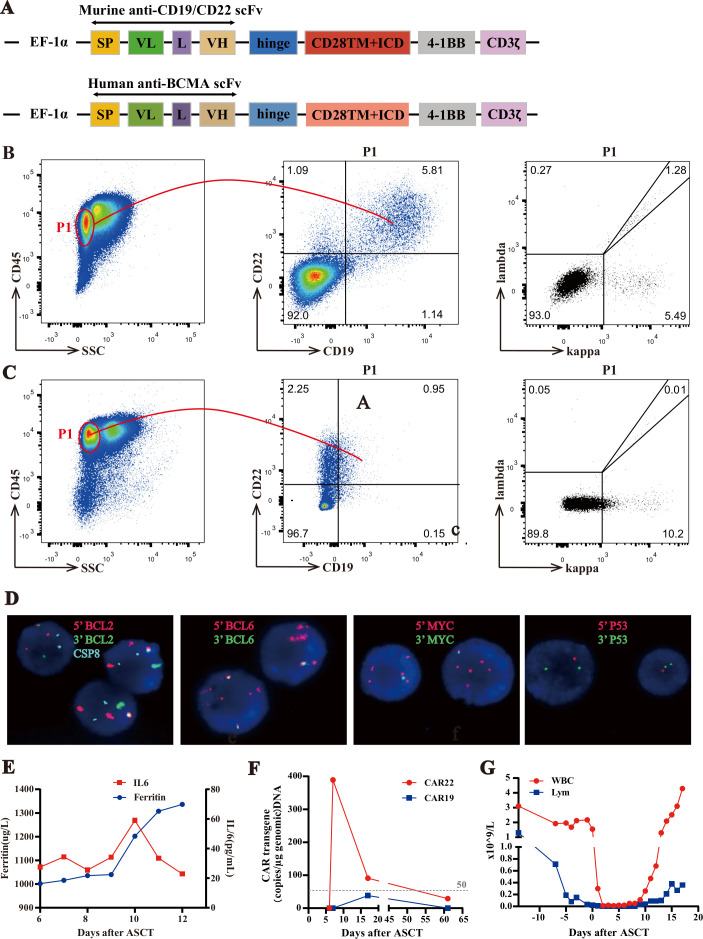
Figure 1.
Clinical examinations in the sequential infusion of murine anti-CD19 and anti-CD22 CAR T cell therapy. (A) Schematic diagram of murine CAR-19/CAR-22 vectors and human CAR-BCMA vectors. (B–C) Phenotypic analysis of ascites before murine CD19 and CD22 CAR T infusion. The P1 gate represented live lymphocytes. In the P1 gate flow cytometry confirmed CD19+ and CD22+ in figure 1F and CD19− and CD22+ in figure 1G, with kappa restriction. (D) Fluorescence in situ hybridization of ascites (BCL2/CSP18 (18q21) probe, BCL6 (3q27) break apart probe, C-MYC (8q24) break apart probe, P53/CSP17 (17p13), 1000×). (E) Levels of IL-6 and ferritin after CAR T cell therapy. (F) CAR-19 and CAR-22 transgene copy numbers detected by ddPCR. (G) Dynamic WCC numbers and lymphocyte numbers before and after CAR T cell therapy. ASCT, autologous hematopoietic stem cell transplantation; CAR, chimeric antigen receptor; IL, interleukin; SP, single peptide; VH, variable H chain; VL, variable L chain; WCC, white cell count.