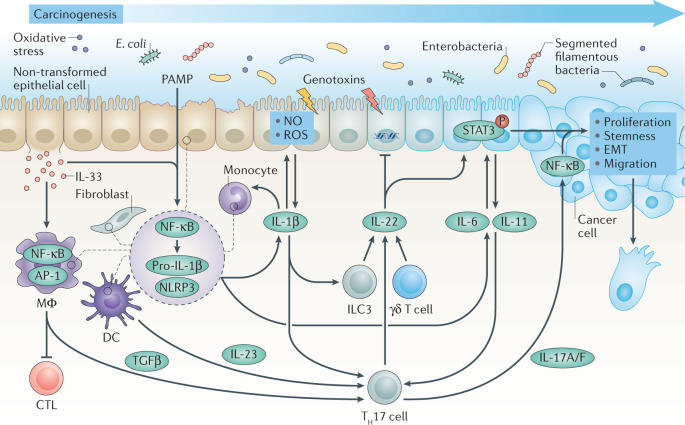
Fig. 1. Interleukins in carcinogenesis.
Persistent inflammation in response to pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns triggers activation of nuclear factor-κB (NF-κB), which primes pro-IL-1β production, and nucleotide-binding oligomerization domain (NOD)-like receptor (NLR) family pyrin domain-containing 3 (NLRP3) inflammasome activation, which causes the release of active IL-1β from fibroblasts, epithelial cells and myeloid cells such as dendritic cells (DCs), monocytes and macrophages (MΦ). In turn, IL-33 derived from tumour-initiating cells recruits macrophages, which upon activator protein 1 (AP-1) signalling produce transforming growth factor-β (TGFβ) that suppresses the function of cytotoxic T lymphocytes (CTLs). IL-1β induces production of nitric oxide (NO) and reactive oxygen species (ROS) by epithelial cells, which may cause DNA damage, and promotes the production of IL-6 and IL-11 from epithelial and myeloid cells, and IL-22 from type 3 innate lymphoid cells (ILC3s) and γδ T cells. Under homeostatic conditions, IL-22 facilitates DNA repair caused by bacterial genotoxins, but in transformed cells, IL-6 and IL-11 together with IL-22 rapidly induce phosphorylation (P) of signal transducer and activator of transcription 3 (STAT3). Activation of STAT3 signalling is observed in multiple types of cancer and induces proliferation, survival, stemness, epithelial–mesenchymal transition (EMT) and migration of transformed cells. IL-1β together with TGFβ induces differentiation of T helper 17 (TH17) cells, which upon IL-23 stimulation from DCs secrete IL-17A and IL-17F (IL-17A/F). IL-17, which typically activates NF-κB to mediate wound-healing signalling, and may exacerbate nascent tumour outgrowth.