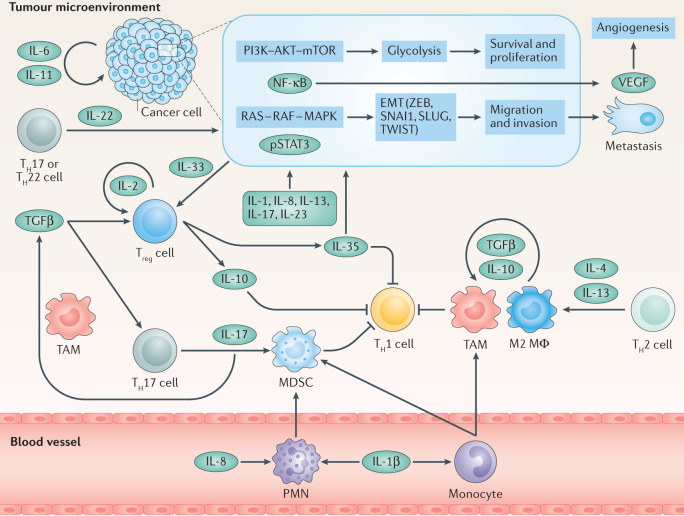
Fig. 2. Tumour microenvironment.
Immune evasion and tumour progression rely on cancer cell-intrinsic and cancer cell-extrinsic cytokine signalling. Several cancer types were demonstrated to overexpress certain cytokines, for example IL-6 or IL-11, which may act in an autocrine manner to activate phosphoinositide 3-kinase (PI3K)–AKT–mTOR signalling to upregulate glycolysis and induce metabolic reprogramming, nuclear factor kappa-κB (NF-κB), rat sarcoma (RAS)–rapidly accelerated fibrosarcoma (RAF)–mitogen-activated protein kinase (MAPK) and signal transducer and activator of transcription 3 (STAT3). These pathways in turn can lead to epithelial–mesenchymal transition (EMT), increased proliferation, reduced apoptosis, increased migration and production of cytokines, such as IL-8, metalloproteinases and vascular endothelial growth factor (VEGF), which induces angiogenesis. Other cytokines, such as IL-1β, IL-13, IL-17, IL-22, IL-23 and IL-35 can also induce EMT and, thus, tumour progression. Tumour-secreted IL-8, in turn, induces recruitment of polymorphonuclear leukocytes (PMNs). Together with monocytes, they differentiate into myeloid-derived suppressor cells (MDSCs), which inhibit T helper 1 (TH1) responses. MDSCs, tumour-associated macrophages (TAMs) and M2 macrophages (MΦ), polarized by TH2-type cytokines, contribute to the pool of transforming growth factor-β (TGFβ) to shape an immunosuppressive microenvironment. In turn, TGFβ together with IL-33 promotes differentiation of regulatory T cells (Treg cells), which bear a high-affinity IL-2 receptor (IL-2R) and are a major source of IL-10 that under chronic conditions suppresses antitumour responses. Alternatively, together with IL-6, TGFβ promotes the differentiation of TH17 cells to produce IL-17 and promote further MDSC recruitment and differentiation. pSTAT3, phosphorylated STAT3; ZEB, zinc-finger E-box-binding homeobox.