Figure 1.
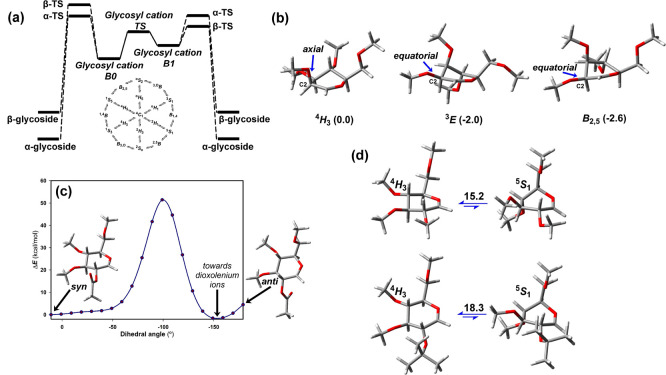
(a) Idealized energy profile for the SN1 mechanism without considering proton transfer reactions. The two-conformer hypothesis of per-O-methyl glycosyl cations is shown. The intermediate B0 geometry is most similar to that of the starting glycoside. The 2D projection shows the interconversion of selected C (chair), B (boat), H (half-chair), and S (skew-boat) conformations. (b) Main conformations and Gibbs free energies of mannopyranosyl cations (4H3, 3E, and B2,5). (c) Single point calculations upon rotation around the H2–C2–O2–CAc torsion. (d) Optimized 4H3 and 5S1 conformations and ΔG differences (kcal/mol) for 2-O-Me and 2-O-isopropyl Glcp. Calculations are carried out at the PBE0-D3/def2-TZVP level (CH2Cl2). Configurations are defined by a three-letter code (Gal, Glc, or Man). Ring size is denoted by p (pyranose).