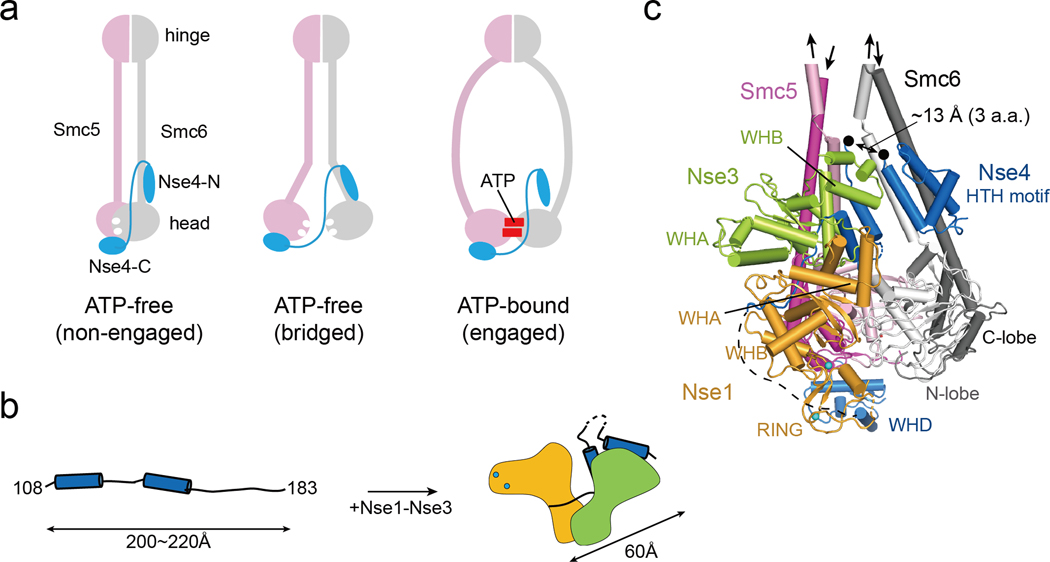
Fig. 7.
A model of Nse1-3-4 engagement with the Smc5/6 head regions
(a) Three possible configurations for Nse4 engagement with the Smc5/6 head regions according to predominant models of SMC complexes. In the absence of ATP, Smc5/6 head regions can adopt the ‘non-engaged’ or ‘bridged’ state. In both states, the head domains are bridged by Nse4 through two interfaces (Smc6-Nse4-N and Smc5-Nse4-C). In ATP-bound state, the Smc6-Nse4-N interface could open.
(b) A cartoon representation of the folding of Nse4 promoted by the Nse1-Nse3 complex. The Nse4M in the absence of Nse1-Nse3 could adopts an extended, string-like structure with a maximum length of 200−220 Å. Nse1-Nse3 shapes Nse4M to a compact (length ~60 Å) form.
(c) Modeling of the Smc5/6 head regions with Nse1-3-4 (details in Fig. S9). In the ATP-free state, the head domains of Smc5 (pink for the N-lobe and magenta for the C-lobe) and Smc6 (white for the N-lobe and gray for the C-lobe) are not engaged, and the coiled-coils of Smc5 and Smc6 are paired. The Nse4 HTH motif and WH domain interact with the neck of Smc6 and the head of Smc5, respectively. A black arrow links the HTH and the Nse4M. Two black dots indicate residues 104 and 108 of Nse4, and the missing three residues between the two dots are expected to span ~13 Å, if we assume that they are fully extended.