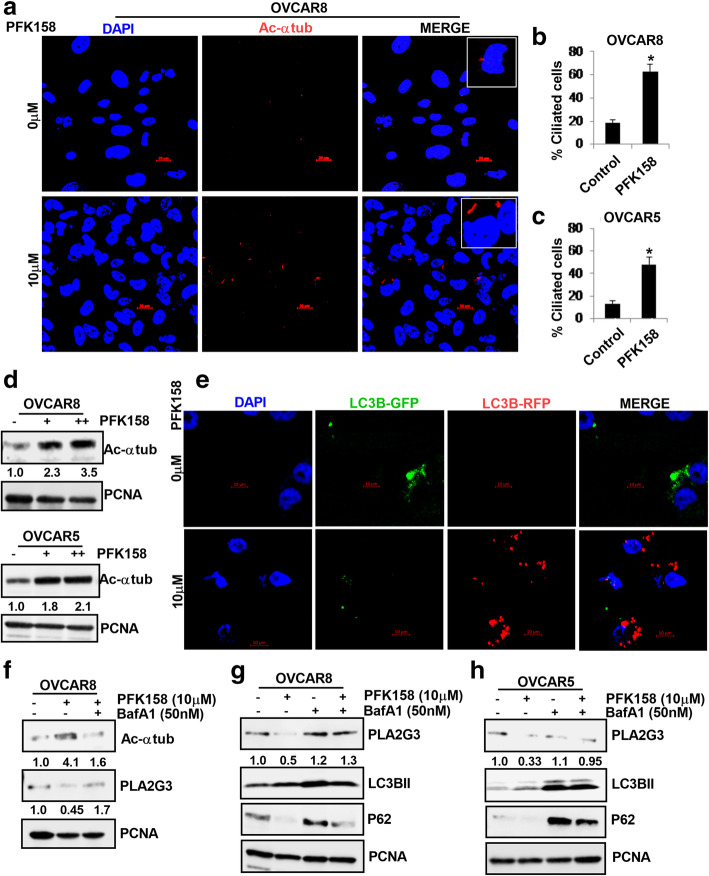
Fig. 5.
PFK158 induces PC by targeting PLA2G3 in an autophagy-dependent manner in OC cells. (A) Representative confocal IF images of fluorescently tagged-acetylated α tubulin (red) indicate percent ciliated cells upon treatment with 10µM PFK158 for 24 h in OVCAR8 cells. Nuclei were stained with DAPI. Scale Bar: 20 μm. (B) In OVCAR8 and (C) OVCAR5 the percent ciliated cells were quantified in 100 cells per field from the IF study and represented as mean ± SD (*p < 0.05 vs. control). (D) Western blot analysis shows the levels of acetylated α-tubulin in both OVCAR8 and 5 cells upon treatment with 5 and 10 µM PFK158 for 24 h. Fold change was calculated using the Image J software, normalized to PCNA endogenous control and provided beneath the panel. (E) IF study of autophagic flux induction (GFP + to RFP + GFP-) in 10µM PFK158 treated OVCAR5 cells for 24 h was performed after transient expression of GFP-RFP-LC3B plasmid. Scale Bar: 10 μm. (F) Immunoblot analysis was performed to show the levels of acetylated α-tubulin and PLA2G3 in the OVCAR8 cells upon treatment with 10µM PFK158 with or without 50nM BafA1 pretreatment (2 h). (G) PLA2G3, LC3BII and p62/SQSTM1 levels were analyzed by western blot in the 10µM PFK158-treated OVCAR8 cells with or without 2 h pretreatment with BafA1 (50nM). (H) Similar analysis was performed in the OVCAR5 cells. PCNA was used as a loading control. Fold change was calculated using the Image J software, normalized to PCNA endogenous control and provided beneath the panel