Abstract
Background
In survivors of sudden cardiac arrest with obstructive coronary artery disease, it remains challenging to distinguish ischemia as a reversible cause from irreversible scar‐related ventricular arrhythmias. We aimed to evaluate the value of implantable cardioverter‐defibrillator (ICD) implantation in sudden cardiac arrest survivors with presumably reversible ischemia and complete revascularization.
Methods and Results
This multicenter retrospective cohort study included 276 patients (80% men, age 67±10 years) receiving ICD implantation for secondary prevention. Angiography was performed before ICD implantation. A subgroup of 166 (60%) patients underwent cardiac magnetic resonance imaging with late gadolinium enhancement before implantation. Patients were divided in 2 groups, (1) ICD‐per‐guideline, including 228 patients with incomplete revascularization or left ventricular ejection fraction ≤35%, and (2) ICD‐off‐label, including 48 patients with complete revascularization and left ventricular ejection fraction >35%. The primary outcome was time to appropriate device therapy (ADT). During 4.0 years (interquartile range, 3.5–4.6) of follow‐up, ADT developed in 15% of the ICD‐off‐label group versus 43% of the ICD‐per‐guideline group. Time to ADT was comparable in the ICD‐off‐label and ICD‐per‐guideline groups (hazard ratio (HR), 0.46; P=0.08). No difference in mortality was observed (HR, 0.95; P=0.93). Independent predictors of ADT included age (HR, 1.03; P=0.01), left ventricular end‐diastolic volume HR, (1.05 per 10 mL increase; P<0.01) and extent of transmural late gadolinium enhancement (HR, 1.12; P=0.04).
Conclusions
This study demonstrates that sudden cardiac arrest survivors with coronary artery disease remain at high risk of recurrent ventricular arrhythmia, even after complete revascularization and with preserved left ventricular function. Late gadolinium enhancement–cardiac magnetic resonance imaging derived left ventricular volumes and extent of myocardial scar were independently associated with.
Keywords: secondary prevention implantable cardioverter defibrillator, sudden cardiac arrest, coronary artery disease, cardiac magnetic resonance, reversible underlying cause
Subject Categories: Sudden Cardiac Death, Secondary Prevention, Myocardial Infarction, Magnetic Resonance Imaging (MRI), Cardiopulmonary Resuscitation and Emergency Cardiac Care
Nonstandard Abbreviations and Acronyms
- ADT
appropriate device therapy
- CTO
chronic total occlusion
- SCA
sudden cardiac arrest
- VA
ventricular arrhythmia
Clinical Perspective
What is New?
Survivors of sudden cardiac arrest with obstructive coronary artery disease and a presumably reversible ischemic cause receiving complete revascularization remain at risk of recurrent ventricular arrhythmias, as 15% of the patients with an off‐label implantable cardioverter defibrillator (ICD) received appropriate device therapy during a median follow‐up of 4 years.
Higher age, increased end‐diastolic volume, and larger extent of transmural late gadolinium enhancement assessed with cardiac magnetic resonance imaging are associated with appropriate device therapy in patients with a secondary prevention ICD with coronary artery disease.
What Are the Clinical Implications?
Patients with sudden cardiac arrest who are considered to be not eligible for secondary prevention ICD implantation according to the current guidelines are still at increased risk of ventricular arrhythmias.
The incidence of appropriate device therapy in sudden cardiac arrest survivors with coronary artery disease and a presumably reversible cause is comparable to the incidence of appropriate device therapy in patients with a primary‐prevention ICD, suggesting that ICD implantation can be justified.
CMR with late gadolinium enhancement provides additional data to estimate the risk of recurrent arrhythmias in patients with a secondary prevention ICD with coronary artery disease; larger left ventricular end‐diastolic volume and extent of transmural LGE are associated with recurrent ventricular arrhythmias.
Guidelines recommend implantable cardioverter‐defibrillator (ICD) implantation for secondary prevention of sudden cardiac death (SCD) in patients with ventricular fibrillation (VF) or hemodynamically unstable ventricular tachycardia (VT) without a reversible cause, regardless of left ventricular ejection fraction (LVEF). 1 Survivors of sudden cardiac arrest (SCA) with a reversible cause, such as electrolyte abnormalities or myocardial ischemia, are considered to be at low risk of recurrent ventricular arrhythmias (VAs) after elimination of the primary cause and are therefore deemed not eligible for ICD implantation. 2 , 3 , 4
A substantial number of patients surviving SCA suffer from obstructive coronary artery disease (CAD). 5 In these patients, it may be challenging to determine whether the arrest was caused by an acute ischemic event that may be restored by coronary revascularization, or by VA related to preexistent irreversible myocardial scar. The physician’s decision whether SCA is primarily triggered by ischemia is typically guided by clinical information, including patient complaints and level of exercise before the arrest, electrocardiographic characteristics, troponin release, and coronary angiographic results. 1 Echocardiography is recommended for functional and structural assessment of the myocardium, whereas additional cardiac magnetic resonance (CMR) imaging with late gadolinium enhancement (LGE) is recommended by current guidelines for noninvasive tissue characterization. 1 , 6 Myocardial scar is an established substrate for VA. 7 Even after exclusion of obstructive CAD by invasive coronary angiography, occult myocardial infarction (MI) is seen on LGE‐CMR in 8% of SCA survivors, 6 emphasizing the difficulty of clinical assessment of substrate reversibility. The same predicament applies in patients with VA caused by myocardial ischemia, treated with complete revascularization but with myocardial scar present on LGE‐CMR. Although guidelines recommend otherwise, recent studies suggest a beneficial effect of secondary prevention ICD in SCA survivors with reversible cause, as these patients remain at high risk for recurrent VA. 8 , 9 , 10 The objectives of this study are to evaluate (1) if SCA survivors with obstructive CAD and a presumably reversible ischemic cause are at lower risk of appropriate device therapy (ADT) compared with survivors with an irreversible ischemic cause, and (2) to evaluate the value of clinical and imaging parameters associated with ADT in SCA survivors with CAD.
Methods
Data Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population and Study Design
This study was designed as a multicenter retrospective observational cohort study. Survivors of SCA attributable to either VT or VF with CAD who received an ICD with or without resynchronization therapy for secondary prevention of SCD between January 2011 and June 2018 were included. The other inclusion criterion was invasive coronary angiography before ICD implantation. The local ethics committee approved data collection and management of this study. Because of the retrospective nature of this study, informed consent was waived by the local medical ethical committee. Clinical and demographic data regarding baseline characteristics were obtained from electronic medical records. Ischemic heart disease was defined as a history of significant obstructive CAD (coronary stenosis of >70% or fractional flow reserve <0.80), a history of MI or prior coronary revascularization with percutaneous coronary intervention or coronary artery bypass grafting, or if patients were diagnosed with CAD at time of cardiac arrest. Patients without ischemic heart disease, without invasive coronary angiography, or who were lost to follow‐up immediately after device implantation were excluded. The study cohort was divided in 2 groups: the ICD‐per‐guideline group consisted of SCA survivors with an irreversible ischemic substrate, defined as (1) incomplete revascularization, including untreated coronary chronic total occlusions (CTOs); (2) history of MI or coronary revascularization without newly developed obstructive coronary lesions at time of SCA; (3) recurrent VA >48 hours after MI; or (4) LVEF ≤35% before ICD implantation, whereas the ICD‐off‐label group consisted of SCA survivors with CAD, treated with complete coronary revascularization (percutaneous coronary intervention or coronary artery bypass grafting) before ICD implantation and LVEF >35%. Two clinical case examples of patients in the ICD‐off‐label group are presented in Figure S1.
CMR With LGE Protocol
LGE‐CMR was performed in a large subgroup of patients (N=166) for either diagnosis of underlying etiology or quantification of left ventricular (LV) function before ICD implantation. Two centers (Amsterdam UMC, Vrije Universiteit Amsterdam, the Netherlands; and Northwest Clinics, Alkmaar, the Netherlands) performed CMR on 1.5 Tesla whole body scanners (Siemens, Erlangen, Germany; and GE Healthcare, Chicago IL), with dedicated phased array cardiac receiver coil. Functional imaging was performed using retrospective ECG‐gated steady‐state free precession cine imaging with breath‐holding in 3 standard long‐axis views and a stack of short‐axis slices covering both ventricles from base to apex. Contrast images were acquired approximately 10 to 15 minutes after intravenous gadolinium administration using a T1‐weighted inversion recovery‐prepared gradient echo sequence with optimized inversion time.
The presence and pattern of gadolinium hyperenhancement were assessed visually. If present, LGE was localized according to the 16‐segment model of the American Heart Association. 11 Ischemic LGE was defined as subendocardial or transmural hyperenhancement in the territory of a coronary artery, and scar transmurality was assessed visually. Subendocardial scar was defined as ≤50% and transmural scar defined as >50% hyperenhancement of LV wall thickness. 12 LV volumes and LVEF were quantified by delineation of the endocardial border at end diastole and end systole from short‐axis cine image stacks, using dedicated software (Mass, Medis, Leiden, the Netherlands; and Argus Function, Siemens, Erlangen, Germany). Papillary muscles were excluded from the myocardium and included in the blood pool.
Ventricular Arrhythmia and Coronary Angiographic Characteristics
Data on level of exercise before SCA were collected and classified as none (eg, sitting), moderate (eg, cycling to work) or extensive exercise (eg, competitive sports). Peak cardiac enzymes, including creatine kinase myocardial band and troponin T, were collected (available only for the patients of the Amsterdam UMC [N=190]). Patients underwent invasive coronary angiography after the index cardiac arrest, with immediate or delayed revascularization if necessary. Multivessel disease was defined as the presence of ≥2 coronary arteries with a significant lesion at time of SCA. CTO was defined as a complete obstruction of a native coronary artery with or without filling through collateral vessels, for a duration of at least 3 months. A patent graft to a preexisting CTO was considered as revascularization of the coronary artery. Successful coronary artery bypass grafting after SCA was considered as complete revascularization therapy.
ICD Eligibility and ICD Settings
Patients were evaluated by a multidisciplinary heart team and accepted for secondary prevention ICD implantation. Patients with an ICD‐off‐label received complete coronary revascularization but were deemed eligible for secondary prevention ICD therapy if preexisting irreversible substrate could not be excluded at the discretion of the treating physician. ICDs were typically programmed with detection rates of >150 beats/min (monitor zone), >182 beats/min (VT 1 zone), and >250 beats/min (VF zone) with extended detection intervals, but could be optimized in the outpatient clinic if this was required. Clinical follow‐up was obtained from regular visits at intervals of 6 months, and from event transmissions of patient devices connected to remote monitoring. Events with antitachycardia pacing and shocks were reviewed by specialized cardiac device technicians and electrophysiologists.
End Points and Follow‐up
The primary end point was time to first ADT. ADT was defined as antitachycardia pacing or shock for VT or VF. Date and type (antitachycardia pacing or shock) of ADT, as well as the cumulative number of ADTs, were collected. Secondary end points were time to all‐cause mortality and VT ablation.
Statistical Analysis
Continuous variables are presented as mean±SD if data were normally distributed or median and interquartile range (IQR) otherwise. Dichotomous and categorical data were expressed as frequencies and percentages. Kaplan–Meier curves, stratified by the 2 groups, were used to visualize differences in time‐to‐event. Median follow‐up time was calculated using reverse Kaplan–Meier analysis. Hazard ratios (HRs) and 95% CIs were obtained using Cox regression. For comparison of the primary end point, patients without ADT were censored at end of follow‐up or time of death. For comparison of overall survival, patients still alive at end of follow‐up were censored at end of follow‐up.
Based on the literature, the following variables were tested as potential confounders or effect modifiers: age, beta‐blocker use, sex, LGE presence, LVEF, and resynchronization therapy. A variable was considered a confounder if the regression coefficient changed by >10%. Effect modification was evaluated by including an interaction term in the model. A variable was considered an effect modifier if the interaction term was statistically significant (P<0.05).
Univariable Cox regression analyses were performed for clinical and imaging variables potentially associated with ADT. Next, variables with a P<0.10 at univariable analysis were entered in a stepwise multivariable Cox regression analysis. Nonsignificant univariable variables were subsequently removed from the multivariable model using a backward elimination procedure. A 2‐sided significance level of 5% was used for all analyses. All statistical analyses were performed using SPSS software package (version 26.0; IBM Corporation, Armonk, NY).
RESULTS
Clinical data of 538 patients with a secondary prevention ICD were collected. Figure 1 presents the consort diagram. A total of 276 patients met the inclusion criteria. Baseline characteristics are described in Table 1, stratified by ICD‐per‐guideline and ICD‐off‐label groups. Mean age was 67±10 years, mean LVEF was 38±11%, and the majority of the patients were men (80%). No significant differences were observed with respect to age, sex, medication use at time of ICD implantation, or device type between both groups.
Figure 1. Consort diagram of the study inclusion process.
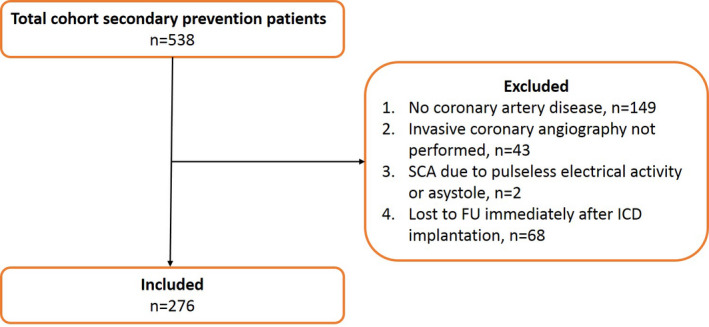
FU indicates follow‐up, ICD, implantable cardioverter defibrillator, and SCA, sudden cardiac arrest.
Table 1.
Baseline Characteristics
| Characteristics | Total Cohort (n=276) | ICD‐per‐Guideline (n=228) | ICD‐Off‐Label (n=48) | P Value |
|---|---|---|---|---|
| Age, y | 67±10 | 67±10 | 65±10 | 0.19 |
| Male sex, n (%) | 242 (80) | 197 (86) | 45 (94) | 0.16 |
| Cardiovascular risk factors | ||||
| Diabetes mellitus, n (%) | 56 (20) | 51 (22) | 5 (10) | 0.06 |
| Hypertension, n (%) | 120 (44) | 98 (52) | 22 (51) | 0.91 |
| Hyperlipidemia, n (%) | 82 (30) | 69 (38) | 13 (32) | 0.43 |
| BMI, kg/m2 | 27±4 | 27±4 | 27±4 | 0.46 |
| Medical history | ||||
| Prior MI, n (%) | 180 (65) | 159 (70) | 21 (44) | <0.01 |
| Prior PCI, n (%) | 90 (33) | 78 (34) | 12 (25) | 0.22 |
| Prior CABG, n (%) | 61 (22) | 58 (25) | 3 (6) | <0.01 |
| LVEF, %* | 38±11 | 36±11 | 48±8 | <0.01 |
| Atrial fibrillation, n (%) | 89 (32) | 79 (35) | 10 (21) | 0.06 |
| Creatinine, µmol/L, median (IQR) | 86 (76–102) | 88 (77–103) | 83 (71–95) | 0.08§ |
| QRS duration, ms, median (IQR) | 108 (96–126) | 110 (96–128) | 98 (91–114) | <0.01§ |
| Cardiac findings and intervention at index event | ||||
| VF at time of OHCA, n (%) | 180 (65) | 143 (63) | 37 (77) | 0.06 |
| CK‐MB†, µg/L | 22 (8–45) | 22 (8–56) | 25 (8–40) | 0.98§ |
| Troponin T‡, µg/L, median (IQR) | 0.33 (0.11–0.98) | 0.32 (0.11–0.95) | 0.37 (0.13–1.00) | 0.72§ |
| Moderate/extensive exercise before arrest, n (%) | 69 (25) | 52 (24) | 17 (36) | 0.08 |
| Diffuse nonobstructive CAD, n (%) | 74 (26) | 73 (32) | 1 (2) | <0.01 |
| Multivessel disease, n (%) | 163 (59) | 133 (58) | 30 (63) | 0.59 |
| CTO present (no prior CABG), n (%) | 100 (36) | 90 (40) | 10 (21) | 0.02 |
| PCI performed, n (%) | 102 (37) | 67 (29) | 35 (73) | <0.001 |
| CABG performed, n (%) | 26 (9) | 12 (5) | 14 (29) | <0.001 |
| Complete revascularization, n (%) | 143 (52) | 95 (42) | 48 (100) | <0.001 |
| Medication at time of ICD implantation | ||||
| ACEi/ARB, n (%) | 237 (86) | 194 (85) | 43 (90) | 0.42 |
| Beta‐blockers, n (%) | 251 (91) | 205 (90) | 46 (96) | 0.27 |
| Calcium channel blocker, n (%) | 23 (8) | 20 (9) | 3 (6) | 0.78 |
| Amiodarone, n (%) | 39 (14) | 35 (15) | 4 (8) | 0.21 |
| Device type | ||||
| ICD, n (%) | 259 (94) | 211 (93) | 48 (100) | 0.50 |
| CRT‐D, n (%) | 17 (6) | 17 (8) | 0 | 0.50 |
Dichotomous variables compared using the chi‐square test or Fischer's exact test. Continuous variables compared using t‐tests unless otherwise indicated. *Based on 199 patients; †Based on N=151; ‡Based on N=156; §Tested using Mann–Whitney U test. ACEi indicates angiotensin‐converting enzyme inhibitors; ARB, angiotensin receptor blocker; BMI, body mass index; CABG, coronary arterial bypass graft surgery; CAD, coronary artery disease; CK‐MB, creatine kinase myocardial band; CRT‐D, resynchronisation therapy defibrillator; CTO, coronary chronic total occlusion; ICD, implantable cardioverter defibrillator; IQR, interquartile region; MI, myocardial infarction; OHCA, out‐of‐hospital cardiac arrest; PCI, percutaneous coronary intervention; and VF, ventricular fibrillation.
Clinical Outcomes
After a median follow‐up of 4.0 years (IQR, 3.5–4.6), ADT occurred in 106 patients (38%), with an incidence rate of 14% per patient‐year. There was no significant difference in follow‐up time between the ICD‐off‐label and ICD‐per‐guideline groups (3.0 years [IQR 2.0–5.0] and 3.0 years [IQR 1.0–4.8], respectively; P=0.22). VA developed in 15% of the ICD‐off‐label group versus 43% of the ICD‐per‐guideline group (Table 2), with an incidence rate of 5% per patient‐year in the ICD‐off‐label group and an incidence rate of 16% per patient‐year in the ICD‐per‐guideline group. Time to ADT was comparable in the ICD‐off‐label group and ICD‐per‐guideline group (HR, 0.46; 95% CI, 0.19–1.11; P=0.08; Figure 2A). The median number of cumulative ADTs per patient was equally distributed between the groups (P=0.57) (Table 2). No difference was observed in time to ADT between patients with LVEF ≤35% compared with patients with LVEF >35% (HR, 1.35; 95% CI, 0.88–2.07; P=0.18). The number of VT ablations was significantly higher in the ICD‐per‐guideline group (P<0.01). A total of 40 patients died during follow‐up (15%), without a significant difference in time to mortality between both groups (HR, 0.95; 95% CI, 0.30–2.98; P=0.93; Figure 2B).
Table 2.
Outcomes
| Parameters | Total Cohort (n=276) | ICD‐per‐Guideline (n=228) | ICD‐Off‐Label (n=48) | P Value |
|---|---|---|---|---|
| ADT, n (%) | 106 (38) | 99 (43) |
7 (15) |
<0.001 |
| Antitachycardia pacing only, n (%) | 63 (23) | 59 (26) | 4 (8) | <0.01 |
| ICD shock, n (%) | 43 (16) | 40 (18) | 3 (6) | 0.05 |
| Cumulative number of ADTs per patient*, n (IQR) | 2 (1–4) | 2 (1–4) | 3 (2–3) | 0.57† |
| VT ablation, n (%) | 27 (10) | 27 (12) | 0 | <0.01 |
| All‐cause mortality, n (%) | 40 (15) | 36 (16) | 4 (8) | 0.18 |
Dichotomous variables compared using the chi‐square test or Fischer's exact test. Continuous variables compared using Mann–Whitney U test. *The cumulative number of ADTs per patient is computed only in patients who received ≥1 ADT; †Tested using the Mann–Whitney U test. ADT indicates appropriate device therapy; ICD, implantable cardioverter defibrillator; IQR, interquartile region; and VT ventricular tachycardia.
Figure 2. The primary and secondary end points during follow‐up.
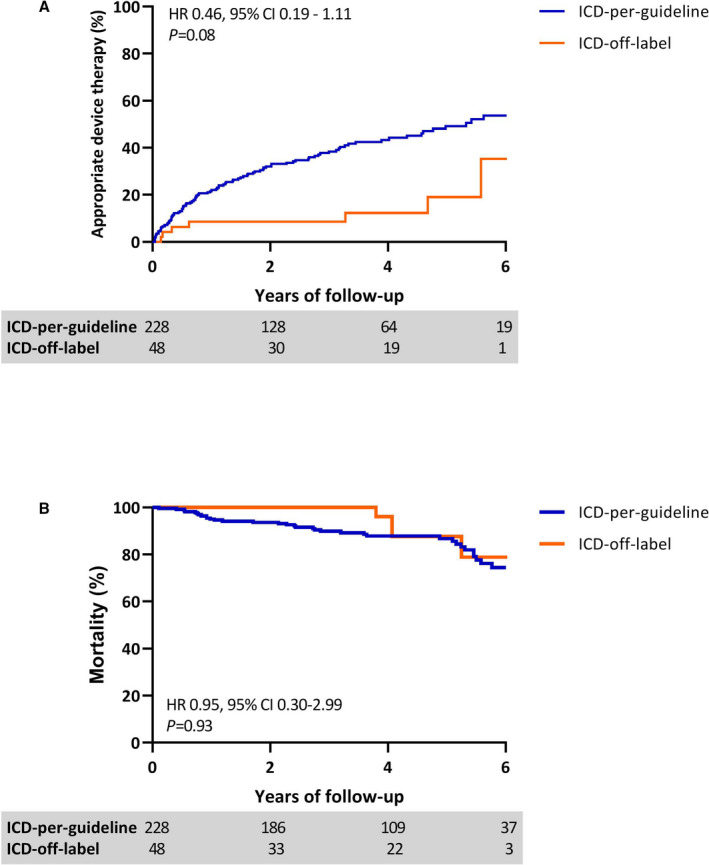
Kaplan–Meier curves depicting differences in (A) appropriate device therapy and (B) all‐cause mortality between the ICD‐per‐guideline and ICD‐off‐label groups during 6 years of follow‐up. Unadjusted values appropriate device therapy: HR, 0.30; 95% CI, 0.14–0.65; P<0.01. Unadjusted values mortality: HR, 0.65; 95% CI, 0.23–1.83; P=0.41. *The HRs in Figure 2 are adjusted for LVEF. HR indicates hazard ratio; and ICD, implantable cardioverter defibrillator.
Circumstances of SCA and Angiographic Findings
VF was the cause of the index arrest in the majority of patients (69%) and almost one‐third of patients performed moderate to extensive exercise at time of SCA (Table 1). Median time between coronary angiography and ICD implantation was 5 days (IQR, 1‐12). Overall, 59% of patients were diagnosed with multivessel CAD, and complete revascularization was performed in 143 (52%) patients. A total of 100 patients (36%) were diagnosed with a CTO at the time of the index event. During hospitalization, 15 (15%) of the patients with CTOs received CTO revascularization before ICD implantation. No differences were observed in sex (P=0.90), mean age (P=0.61), or mean LVEF (P=0.69) between patients with a CTO and without a CTO at index event (Table S1). There was no difference in time to ADT between patients with an untreated CTO compared with patients without a CTO or treated CTO (HR 1.34; 95% CI 0.88‐2.02; P=0.17; Figure 3A). However, in patients with incomplete revascularization (including untreated CTO), time to ADT was shorter compared with patients with complete coronary revascularization (HR 1.54; 95% CI 1.05–2.26; P=0.03; Figure 3B). In the ICD‐off‐label group, VF at index event was associated with less ADT, whereas VT at the index event was associated with more ADT during follow‐up (P=0.04). Creatine kinase myocardial band values (P=0.73), troponin T values (P=0.22), and moderate to extensive exercise before SCA (P>0.99) were not associated with ADT at follow‐up.
Figure 3. The value of CTO and revascularization on appropriate device therapy during follow‐up.
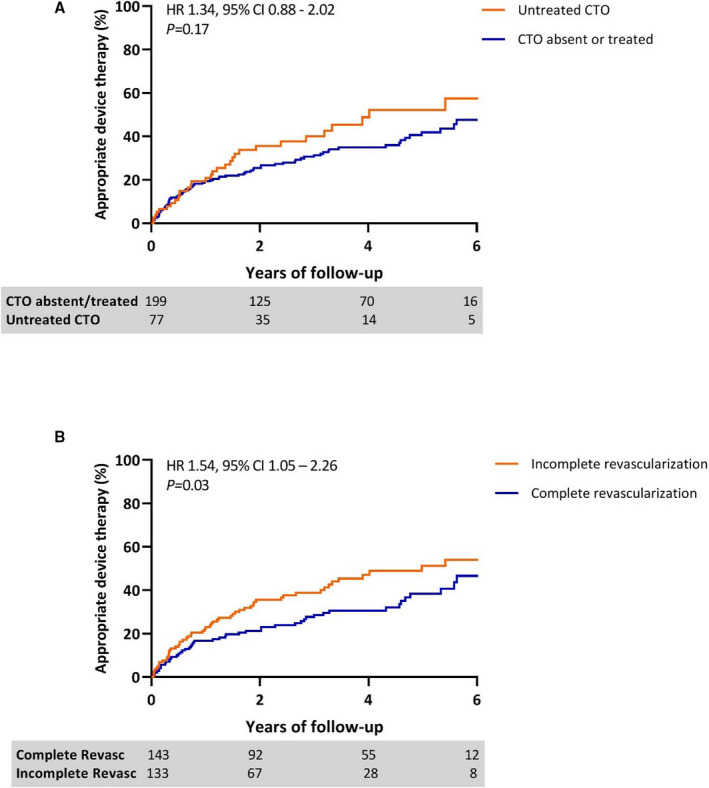
Kaplan–Meier curves depicting differences in appropriate device therapy in (A) patients with or without CTO present, and in (B) patients with complete versus incomplete revascularization during 6 years of follow‐up. CTO indicates chronic total occlusion; ICD, implantable cardioverter defibrillator; and HR, hazard ratio.
CMR Characteristics
A total of 166 patients received CMR with LGE imaging before ICD implantation (60% of the total cohort). Patients who received CMR were younger and were more often diagnosed with single‐vessel CAD. There were no differences with respect to sex, LVEF, prior MI, or end points in patients with and without CMR (Table S2). Median time interval between CMR and ICD implantation was 6 days (IQR 3‐13). Table 3 depicts the CMR results, stratified by both groups. The majority of patients demonstrated LGE on CMR (93%), primarily with an ischemic pattern (90%) and with a median extent of 5 (IQR 3‐7) segments. LV volumes were significantly larger in the ICD‐per‐guideline group compared with the ICD‐off‐label group (eg, LV end‐diastolic volume [LVEDV] 240±84 mL versus 197±45 mL, respectively; P<0.01), and LVEF was significantly lower (36±22% versus 48±8%, respectively; P<0.01). There was no significant difference between the extent of LGE in the ICD‐per‐guideline group and the ICD‐off‐label group, although the extent of LGE with >50% transmurality tended to be larger in the ICD‐per‐guideline group (3 [IQR, 1‐5] segments versus 2 [IQR, 0–4] segments; P=0.13, respectively). Table S3 presents the outcome of the ICD‐per‐guideline group and ICD‐off‐label group with CMR.
Table 3.
CMR Values
| Cohort With CMR (n=166) | ICD‐per‐Guideline with CMR (n=134) | ICD‐Off‐Label With CMR (n=32) | P Value | |
|---|---|---|---|---|
| End‐diastolic volume, mL | 231±80 | 240±84 | 197±45 | <0.01 |
| End‐systolic volume, mL | 147±73 | 157±76 | 104±35 | <0.01 |
| LVEF, % | 39±12 | 36±22 | 48±8 | <0.01 |
| LVEF ≥50%, n (%) | 31 (19) | 17 (13) | 14 (44) | <0.01 |
| Any LGE present, n (%) | 155 (93) | 127 (95) | 28 (88) | 0.23 |
| Presence ischemic LGE, n (%) | 149 (90) | 123 (92) | 26 (81) | 0.32 |
| No. of segments with ischemic LGE, median n (IQR) | 5 (3–7) | 5 (3–7) | 5 (3–6) | 0.36* |
| No. transmural segments (LGE >50%), median n (IQR) | 3 (1–5) | 3 (1–5) | 2 (0–4) | 0.13* |
| No. subendocardial segments, median n (IQR) | 1 (0‐3) | 1 (0‐3) | 1 (0‐4) | 0.59* |
Dichotomous variables compared using the chi‐square test or Fischer's exact test. Continuous variables compared using t‐tests unless otherwise indicated. *Tested using the Mann–Whitney U test. CMR indicates cardiac magnetic resonance; ICD, implantable cardioverter defibrillator; IQR, interquartile region; LGE, late gadolinium enhancement; and LVEF, left ventricular ejection fraction.
Parameters Associated With ADT
Table 4 presents the univariable and multivariable analyses of clinical and imaging parameters for the association with time to ADT in patients receiving LGE‐CMR imaging (N=166). Parameters associated with time to ADT at univariable analysis were higher age (P=0.02), male sex (P=0.08), prior MI (P=0.04), larger LVEDV (P<0.01), lower LVEF (P=0.01), number of segments with ischemic LGE with >50% transmurality (P<0.01), incomplete revascularization (P=0.05), untreated CTO (P=0.09), and an ICD‐per‐guideline indication (P=0.04). Interestingly, LGE presence was not significantly associated with time to ADT (P=0.17). Multivariable Cox regression analyses using backward selection showed that higher age (P=0.02), larger LVEDV (P<0.01), and higher number of segments with LGE with >50% transmurality (P=0.04) remained independently associated with time to ADT. Univariable and multivariable analyses without imaging parameters of the total study cohort (N=276) are presented in Table S4.
Table 4.
Univariable and Multivariable Cox Regression Analysis of Clinical and Imaging Parameters for Predicting Appropriate Device Therapy in Patients Receiving CMR
| Parameter | HR (95% CI) | P Value | HR (95% CI) | P Value |
|---|---|---|---|---|
| Univariable analysis | Multivariable analysis | |||
| Age (years)* | 1.03 (1.00–1.06) | 0.02 | 1.04 (1.01–1.06) | 0.01 |
| Male sex* | 2.49 (0.90–6.86) | 0.08 | … | … |
| Diabetes mellitus* | 0.93 (0.49–1.79) | 0.83 | ||
| Prior MI* | 1.88 (1.04–3.42) | 0.04 | … | … |
| Atrial fibrillation* | 0.88 (0.50–1.56) | 0.67 | ||
| Beta‐blocking therapy* | 0.88 (0.35–2.21) | 0.79 | ||
| Creatinine (µmol/L)† | 1.00 (0.99–1.01) | 0.75 | ||
| LVEF per 10%‡ | 0.76 (0.62–0.94) | 0.01 | … | … |
| LVEDV per 10 mL§ | 1.05 (1.02–1.08) | <0.01 | 1.06 (1.02–1.09) | <0.01 |
| LGE presence* | 2.66 (0.65–10.91) | 0.17 | ||
| No. transmural LGE segments* | 1.17 (1.06–1.29) | <0.01 | 1.12 (1.01–1.25) | 0.04 |
| Moderate‐intensive exercise* | 1.14 (0.66–1.95) | 0.64 | ||
| CK‐MB|| | 1.00 (0.99–1.00) | 0.68 | ||
| Multivessel CAD* | 1.13 (0.68–1.85) | 0.64 | ||
| Incomplete revascularization* | 1.67 (1.01–2.76) | 0.05 | … | … |
| CTO treated conservatively* | 1.57 (0.94–2.64) | 0.09 | … | … |
| ICD‐per‐guideline indication* | 2.48 (1.07–5.76) | 0.04 | … | … |
| CRT‐D* | 1.79 (0.71–4.47) | 0.22 | ||
Based on 166 patients; †Based on N=158; ‡Based on N=162; §Based on N=165; ||Based on N=106. CAD, coronary artery disease; CK‐MB, creatinine kinase myocardial band; CMR, cardiac magnetic resonance; CRT‐D, resynchronization therapy defibrillator; CTO, coronary chronic total occlusion, ICD, implantable cardioverter defibrillator; LGE, late gadolinium enhancement; LVEDV, left ventricular end diastolic volume; LVEF, left ventricular ejection fraction; and MI, myocardial infarction.
DISCUSSION
This study shows that survivors of SCA with CAD remain at high risk of recurrent VA during follow‐up. Even patients with a presumably reversible ischemic cause, with LVEF >35% and complete revascularization, remain at risk of recurrent VA. SCA survivors with CAD with increased LVEDV and high number of segments with LGE with >50% transmurality are at high risk of ADT. In addition, this study suggests that incomplete revascularization increases the risk of ADT, emphasizing the need to strive for complete revascularization before ICD implantation.
Reversible Causes and ICD Eligibility
ICD implantation is an established therapy to improve survival rates of SCA, but SCA survivors with a reversible and correctable cause are not eligible for ICD therapy according to current guidelines. 1 However, this recommendation is based on a paucity of data, since previous randomized secondary prevention ICD trials excluded SCA survivors with reversible causes. 2 , 3 , 4 The present study demonstrates that patients with a secondary prevention ICD with CAD and a preserved LVEF and complete revascularization, suggesting removal of the identifiable underlying cause, remain at increased risk of VA during follow‐up. This indicates that ICD implantation might be justified in this group of patients. Concerning the recurrence of arrhythmias in SCA survivors with CAD, previous studies found conflicting results. Several studies reported a negligible benefit of ICD therapy in this subgroup, 8 , 13 whereas others are in line with the current study and report that SCA survivors with CAD remain at risk of VA after complete revascularization and may benefit from ICD implantation. 9 , 14 , 15 This inconsistency in results is not well elucidated. Several studies included a substantial group of patients with a nonshockable rhythm at the index event, 8 , 13 in whom the expected beneficial effect of ICD therapy might be reduced. Moreover, differences in primary outcome measure, including all‐cause mortality, 13 , 14 cardiac mortality, 9 and ADT, 8 , 15 might explain the ambiguous results. The AVID (Antiarrhythmic Versus Implantable Defibrillators) registry, including patients screened but deemed not eligible for the AVID trial, indicated that mortality rates were higher in SCA survivors with a reversible cause compared with patients with an irreversible cause. 14 Although the current study shows that the ADT rate was lower in the ICD‐off‐label group compared with the ICD‐per‐guideline group (15% versus 43% after 4 years of follow‐up), the event rate of ADT in the ICD‐off‐label group is comparable to event rates seen in the current primary prevention ICD population. Primary prevention ICD registries reported ADT in approximately 10% to 20% of patients during 3 years of follow‐up, 16 , 17 suggesting that secondary prevention ICD implantation based on reversible ischemic causes is justifiable.
It can be argued that the underlying pathophysiology of CAD is not entirely correctible or reversible by revascularization of the obstructive (culprit) lesion, since CAD has a progressive nature. Although percutaneous coronary intervention would temporarily resolve a critical coronary stenosis, new lesions may develop over time. 18 Furthermore, studies have shown an important genetic predisposition of developing VA during ischemia in MI. A familial history of SCD was an independent risk factor for VA in MI. 19 , 20 This suggests that patients developing VF or VT during an ischemic event are at increased risk of SCD when new ischemia occurs at follow‐up.
Value of CMR
This study demonstrates that increased LV volume, expressed as LVEDV, is independently associated with ADT, which is in line with previous reports. 21 LV dilation increases wall stress, which was found to increase the risk of VA. 22 , 23 Interestingly, LVEF, currently used in guidelines for risk assessment of VA, is in the present study only univariably associated with ADT. The current study shows that the extent of LGE with >50% transmurality is also independently associated with the primary end point. Myocardial scar is an established substrate for VA, 6 and previous studies demonstrated that additional CMR with LGE imaging was able to detect myocardial scar in the majority of SCA survivors, 24 , 25 even after exclusion of obstructive CAD. 6 A recently published consensus paper on risk assessment of arrhythmias recommends LGE‐CMR for imaging of myocardial scar. 26
Scar after an ischemic event is typically seen in a subendocardial or transmural pattern. 27 LGE transmurality of >50% of wall thickness is in general considered as nonviable, based on in previous studies in which LGE >50% was associated with no or limited functional recovery after revascularization. 12 However, this may have affected the choice of a conservative treatment in patients with incomplete revascularization or untreated CTO in the present cohort. Although the infarcted segments were classified as nonviable based on LGE transmurality, viable myocardium with electrical activity can still be present in the border zone and the residual noninfarcted myocardial wall. Previous studies demonstrated a significant association between border zone and VA. 28 , 29 The continuing ischemia in the residual viable myocardium and border zone attributable to untreated coronary stenosis can cause recurrence of VA. 30 The results of the present study suggest that complete coronary revascularization might reduce the burden of VA, as revascularization decreases the ischemic burden and therewith the associated electrical instability and VAs, irrespective of scar extent and potential functional recovery.
Limitations
Several limitations should be kept in mind when interpreting these data. First, this is a retrospective observational study suffering from the biases inherent to this design. Moreover, the study population is rather small, and results should therefore be interpreted with caution. Second, selection of patients in the ICD‐off‐label group is biased by indication, since the clinical decision to implant an off‐label ICD for secondary prevention in this group was based on expert opinion. However, in both clinics, every patient is discussed in a dedicated heart team meeting before acceptance for ICD implantation. Unfortunately, a control group including SCA survivors with CAD without an ICD for secondary prevention was not available for comparison. Third, in the assessment of myocardial substrate, T2‐weighted CMR imaging may be valuable, since it visualizes myocardial edema. The presence of edema suggests recent myocardial injury, 24 allowing differentiation of newly developed scar from preexisting old substrate. However, this was not routinely performed, and data are therefore missing in the current evaluation. The value of distinguishing recent myocardial damage with edema from old preexisting myocardial scar on the prognosis in patients with CAD after SCA may be of interest for future studies. Fourth, the value of echocardiography was not analyzed in this study, as only a minority of patients received a complete echocardiography examination including LVEF quantification.
CONCLUSIONS
In conclusion, survivors of SCA with CAD are at high risk of recurrent VA during follow‐up. Even in patients with a presumably reversible ischemic cause, with complete revascularization and LVEF ≥35%, the risk of recurrent VA is substantial. Hence, secondary prevention ICD therapy might be justified in this off‐label patient subgroup, since the event rate of ADT is comparable to the event rate seen in the primary prevention ICD population. Moreover, the current study shows that higher age, larger LVEDV, and increased extent of LGE with >50% transmurality are independent predictors for recurrence of VA, suggesting that LGE‐CMR should be incorporated in a patient‐guided risk assessment for survivors of SCA. Since our study and other observational studies are suggesting that SCA patients with a reversible cause based on CAD might benefit from ICD therapy, a randomized study is required to further clarify this vexing problem.
Sources of Funding
None.
Disclosures
None.
Supporting information
Tables S1–S4
Figure S1
Acknowledgments
We are grateful for the assistance with the statistics in this report of Peter M. van de Ven (assistant professor biostatistics, Amsterdam UMC, Vrije Universiteit Amsterdam, Department of Epidemiology and Data Science).
(J Am Heart Assoc. 2021;10:e019101. DOI: 10.1161/JAHA.120.019101.)
For Sources of Funding and Disclosures, see page 10.
References
- 1. Priori SG, Blomstrom‐Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and. Congenital Cardiology (AEPC). Eur Heart J. 2015;36:2793–2867. [DOI] [PubMed] [Google Scholar]
- 2. Kuck KH, Cappato R, Siebels J, Ruppel R. Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest: the Cardiac Arrest Study Hamburg (CASH). Circulation. 2000;102:748–754. [DOI] [PubMed] [Google Scholar]
- 3. Connolly SJ, Gent M, Roberts RS, Dorian P, Roy D, Sheldon RS, Mitchell LB, Green MS, Klein GJ, O'Brien B. Canadian implantable defibrillator study (CIDS): a randomized trial of the implantable cardioverter defibrillator against amiodarone. Circulation. 2000;101:1297–1302. [DOI] [PubMed] [Google Scholar]
- 4. The Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators . Antiarrhythmics versus Implantable Defibrillators I. A comparison of antiarrhythmic‐drug therapy with implantable defibrillators in patients resuscitated from near‐fatal ventricular arrhythmias. N Engl J Med 1997;337:1576–1583. [DOI] [PubMed] [Google Scholar]
- 5. Myerburg RJ, Kessler KM, Castellanos A. Sudden cardiac death: structure, function, and time‐dependence of risk. Circulation. 1992;85:I2–10. [PubMed] [Google Scholar]
- 6. Rodrigues P, Joshi A, Williams H, Westwood M, Petersen SE, Zemrak F, Schilling RJ, Kirkby C, Wragg A, Manisty C, et al. Diagnosis and prognosis in sudden cardiac arrest survivors without coronary artery disease: utility of a clinical approach using cardiac magnetic resonance imaging. Circ Cardiovasc Imaging. 2017;10:e006709. [DOI] [PubMed] [Google Scholar]
- 7. Scott PA, Morgan JM, Carroll N, Murday DC, Roberts PR, Peebles CR, Harden SP, Curzen NP. The extent of left ventricular scar quantified by late gadolinium enhancement MRI is associated with spontaneous ventricular arrhythmias in patients with coronary artery disease and implantable cardioverter‐defibrillators. Circ Arrhythm Electrophysiol. 2011;4:324–330. [DOI] [PubMed] [Google Scholar]
- 8. Gupta A, Pasupula DK, Bhonsale A, Kancharla K, Wang NC, Adelstein E, Jain S, Saba S. Implantable cardioverter‐defibrillator therapy in device recipients who survived a cardiac arrest associated with a reversible cause. J Cardiovasc Electrophysiol. 2018;29:1413–1417. [DOI] [PubMed] [Google Scholar]
- 9. Madhavan M, Friedman PA, Lennon RJ, Prasad A, White RD, Sriram CS, Gulati R, Gersh BJ. Implantable cardioverter‐defibrillator therapy in patients with ventricular fibrillation out of hospital cardiac arrest secondary to acute coronary syndrome. J Am Heart Assoc. 2015;4:e001255. DOI: 10.1161/JAHA.114.001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Winther‐Jensen M, Kjaergaard J, Lassen JF, Kober L, Torp‐Pedersen C, Hansen SM, Lippert F, Kragholm K, Christensen EF, Hassager C. Implantable cardioverter defibrillator and survival after out‐of‐hospital cardiac arrest due to acute myocardial infarction in Denmark in the years 2001–2012, a nationwide study. Eur Heart J Acute Cardiovasc Care. 2017;6:144–154. [DOI] [PubMed] [Google Scholar]
- 11. Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS, et al. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. [DOI] [PubMed] [Google Scholar]
- 12. Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM. The use of contrast‐enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–1453. DOI: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 13. Ladejobi A, Pasupula DK, Adhikari S, Javed A, Durrani AF, Patil S, Qin D, Ahmad S, Munir MB, Rijal S, et al. Implantable defibrillator therapy in cardiac arrest survivors with a reversible cause. Circ Arrhythm Electrophysiol. 2018;11:e005940. DOI: 10.1161/CIRCEP.117.005940. [DOI] [PubMed] [Google Scholar]
- 14. Wyse DG, Friedman PL, Brodsky MA, Beckman KJ, Carlson MD, Curtis AB, Hallstrom AP, Raitt MH, Wilkoff BL, Greene HL, et al. Life‐threatening ventricular arrhythmias due to transient or correctable causes: high risk for death in follow‐up. J Am Coll Cardiol. 2001;38:1718–1724. [DOI] [PubMed] [Google Scholar]
- 15. van Dijk VF, Quast ABE, Schaap J, Balt JC, Kelder JC, Wijffels M, de Groot JR, Boersma LVA. ICD implantation for secondary prevention in patients with ventricular arrhythmia in the setting of acute cardiac ischemia and a history of myocardial infarction. J Cardiovasc Electrophysiol. 2020;31:536–543. [DOI] [PubMed] [Google Scholar]
- 16. Almehmadi F, Porta‐Sanchez A, Ha ACT, Fischer HD, Wang X, Austin PC, Lee DS, Nanthakumar K. Mortality implications of appropriate implantable cardioverter defibrillator therapy in secondary prevention patients: contrasting mortality in primary prevention patients from a prospective population‐based registry. J Am Heart Assoc. 2017;6:e006220. DOI: 10.1161/JAHA.117.006220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Providência R, Boveda S, Defaye P, Segal O, Algalarrondo V, Sadoul N, Lambiase P, Piot O, Klug D, Perier M‐C, et al. Outcome of primary prevention implantable cardioverter defibrillator therapy according to New York Heart Association functional classification. Am J Cardiol. 2016;118:1225–1232. DOI: 10.1016/j.amjcard.2016.07.037. [DOI] [PubMed] [Google Scholar]
- 18. Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111:3481–3488. [DOI] [PubMed] [Google Scholar]
- 19. Adabag AS, Luepker RV, Roger VL, Gersh BJ. Sudden cardiac death: epidemiology and risk factors. Nat Rev Cardiol. 2010;7:216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dekker LRC, Bezzina CR, Henriques JPS, Tanck MW, Koch KT, Alings MW, Arnold AER, de Boer M‐J, Gorgels APM, Michels HR, et al. Familial sudden death is an important risk factor for primary ventricular fibrillation: a case‐control study in acute myocardial infarction patients. Circulation. 2006;114:1140–1145. DOI: 10.1161/CIRCULATIONAHA.105.606145. [DOI] [PubMed] [Google Scholar]
- 21. Takano T, Tanaka K, Ozaki K, Sato A, Iijima K, Yanagawa T, Izumi D, Ozawa T, Fuse K, Sato M, et al. Clinical predictors of recurrent ventricular arrhythmias in secondary prevention implantable cardioverter defibrillator recipients with coronary artery disease‐ lower left ventricular ejection fraction and incomplete revascularization. Circ J. 2018;82:3037–3043. [DOI] [PubMed] [Google Scholar]
- 22. James MA, MacConnell TJ, Jones JV. Is ventricular wall stress rather than left ventricular hypertrophy an important contributory factor to sudden cardiac death? Clin Cardiol. 1995;18:61–65. DOI: 10.1002/clc.4960180205. [DOI] [PubMed] [Google Scholar]
- 23. Sogaard P, Gotzsche CO, Ravkilde J, Norgaard A, Thygesen K. Ventricular arrhythmias in the acute and chronic phases after acute myocardial infarction. Effect of intervention with captopril. Circulation. 1994;90:101–107. [DOI] [PubMed] [Google Scholar]
- 24. Andreini D, Dello Russo A, Pontone G, Mushtaq S, Conte E, Perchinunno M, Guglielmo M, Coutinho Santos A, Magatelli M, Baggiano A, et al. CMR for identifying the substrate of ventricular arrhythmia in patients with normal echocardiography. JACC Cardiovasc Imaging. 2020;13:410–421. DOI: 10.1016/j.jcmg.2019.04.023. [DOI] [PubMed] [Google Scholar]
- 25. White JA, Fine NM, Gula L, Yee R, Skanes A, Klein G, Leong‐Sit P, Warren H, Thompson T, Drangova M, et al. Utility of cardiovascular magnetic resonance in identifying substrate for malignant ventricular arrhythmias. Circ Cardiovasc Imaging. 2012;5:12–20. DOI: 10.1161/CIRCIMAGING.111.966085. [DOI] [PubMed] [Google Scholar]
- 26. Nielsen JC, Lin YJ, de Oliveira Figueiredo MJ, Sepehri Shamloo A, Alfie A, Boveda S, Dagres N, Di Toro D, Eckhardt LL, Ellenbogen K, et al. European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) expert consensus on risk assessment in cardiac arrhythmias: use the right tool for the right outcome, in the right population. Europace. 2020;36:553–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McCrohon JA, Moon JC, Prasad SK, McKenna WJ, Lorenz CH, Coats AJ, Pennell DJ. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium‐enhanced cardiovascular magnetic resonance. Circulation. 2003;108:54–59. [DOI] [PubMed] [Google Scholar]
- 28. Schmidt André, Azevedo CF, Cheng A, Gupta SN, Bluemke DA, Foo TK, Gerstenblith G, Weiss RG, Marbán E, Tomaselli GF, et al. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation. 2007;115:2006–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rijnierse MT, van der Lingen A‐L, de Haan S, Becker MAJ, Harms HJ, Huisman MC, Lammertsma AA, van de Ven PM, van Rossum AC, Knaapen P, et al. Value of CMR and PET in predicting ventricular arrhythmias in ischemic cardiomyopathy patients eligible for ICD. JACC Cardiovasc Imaging. 2020;13:1755–1766. [DOI] [PubMed] [Google Scholar]
- 30. Nombela‐Franco L, Iannaccone M, Anguera I, Amat‐Santos IJ, Sanchez‐Garcia M, Bautista D, Calvelo MN, Di Marco A, Moretti C, Pozzi R, et al. Impact of chronic total coronary occlusion on recurrence of ventricular arrhythmias in ischemic secondary prevention implantable cardioverter‐defibrillator recipients (VACTO Secondary Study): Insights from coronary angiogram and electrogram analysis. JACC Cardiovasc Interv. 2017;10:879–888. DOI: 10.1016/j.jcin.2017.02.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4
Figure S1


