Abstract
Background
Physical inactivity and low cardiorespiratory fitness (CRF) are associated with higher risk of heart failure. However, the independent contributions of objectively measured sedentary time, physical activity, and CRF toward left ventricular (LV) structure and function are not well established.
Methods and Results
We included 1368 participants from the DHS (Dallas Heart Study) (age, 49 years; 40% men) free of cardiovascular disease who had physical activity and sedentary time measured by accelerometer, CRF estimated from submaximal treadmill test, and cardiac magnetic resonance imaging performed using 3‐T magnetic resonance imaging. A series of linear regression models were constructed to evaluate the associations of sedentary time, moderate physical activity, vigorous physical activity, and CRF with LV parameters after adjustment for established cardiovascular risk factors. We observed a modest correlation between CRF levels and objectively measured moderate (correlation coefficient, 0.17; P<0.001) and vigorous physical activity (correlation coefficient, 0.25; P<0.001) levels. In contrast, sedentary time was not associated with CRF. In adjusted analysis, both vigorous physical activity and higher CRF were significantly associated with greater stroke volume, LV mass, LV end‐diastolic volume, and lower arterial elastance, independent of other confounders. Sedentary time and moderate physical activity levels were not associated with LV parameters.
Conclusions
Vigorous physical activity and CRF are significantly associated with cardiac structure and function parameters. Future studies are needed to determine if interventions aimed at improving CRF levels may favorably modify cardiac structure and function.
Keywords: cardiac function, cardiac remodeling, physical exercise
Subject Categories: Lifestyle, Exercise Testing, Remodeling
Nonstandard Abbreviations and Acronyms
- CCLS
Cooper Center Longitudinal Study
- CPM
counts per minute
- CRF
cardiorespiratory fitness
- DHS
Dallas Heart Study
- MHR
maximum heart rate
- SV
stroke volume
- VO2
oxygen consumption uptake
Clinical Perspective
What Is New?
The associations of objectively measured physical activity, cardiorespiratory fitness, and sedentary time with measures of cardiac structure and function are not well understood.
Higher levels of cardiorespiratory fitness and vigorous physical activity are associated with favorable cardiac structure and function.
What Are the Clinical Implications?
Low levels of cardiorespiratory fitness and physical activity may contribute to downstream risk of heart failure through adverse effects on cardiac structure and function.
Interventions aimed at improving physical activity and cardiorespiratory fitness need to be evaluated for their effects on cardiac structure and function and downstream risk of heart failure.
Heart failure (HF) is an important cardiovascular comorbidity among older individuals and associated with high burden of morbidity and mortality. 1 Physical inactivity and low levels of cardiorespiratory fitness (CRF) are well‐established risk factors for HF. 2 , 3 , 4 , 5 , 6 , 7 Recent studies have also identified excess levels of sedentary time as a significant risk factor for HF, independent of physical activity and other potential confounders. 8 However, the mechanism by which physical activity, sedentary time, and CRF may modify risk of HF is not well understood. Furthermore, the contributions of physical inactivity and sedentary behavior toward HF development, independent of CRF levels, are not well known.
Prior studies have identified distinct subclinical cardiac phenotypes in the pathway of progression from at‐risk to clinical HF. Specifically, subclinical abnormalities in left ventricular (LV) function have been associated with higher risk of HF. 9 , 10 , 11 Higher LV mass and LV hypertrophy, particularly in the presence of subclinical myocardial injury (malignant LV hypertrophy), have also been associated with higher risk of HF. 12 In contrast, physiologic LV hypertrophy, as seen in athletes, and LV hypertrophy in absence of myocardial injury have not been associated with abnormalities in LV function, compliance, or risk of HF. 13 , 14 Prior studies have also linked low self‐reported physical activity and high sedentary time with adverse LV remodeling and subclinical LV systolic and diastolic dysfunction. 15 However, these studies were limited by use of self‐reported measures of physical activity and sedentary time and the lack of data on CRF. The independent associations of objectively measured physical activity, sedentary time, and CRF levels with these intermediate cardiac phenotypes are not well defined. This represents an important knowledge gap in our understanding of how these lifestyle factors may modify the risk of HF. Accordingly, we evaluated the independent associations of objectively measured physical activity, sedentary time, and CRF levels with measures of cardiac structure and function among participants of the DHS (Dallas Heart Study).
Methods
The data, methods used in the analysis, and materials used to conduct the research will not be made available to any researcher for purposes of reproducing the results or replicating the procedure.
Study Population
The DHS is a probability‐based, multiethnic cohort study of Dallas county residents that intentionally oversampled Black individuals in efforts to enroll an equal percentage of Black and non‐Black study participants. Race/ethnicity was self‐reported by participants per US Census categories. DHS recruitment and design have been published previously. 16 The original DHS participants, enrolled from 2000 to 2002, and their spouses were invited for a follow‐up examination in 2007 to 2009, known as DHS phase 2 (DHS‐2). DHS‐2 included 3401 study participants, of which 51% were non‐Hispanic Black participants. Study participants underwent a comprehensive health examination, including measurement of blood pressure, collection of blood and urine samples, cardiac magnetic resonance imaging (CMR), accelerometry, and CRF testing.
Among 3401 participants of the DHS‐2, 2771 had CRF assessment data available. For the present study, we excluded participants with history of myocardial infarction, HF, or cardiac arrest, missing CMR data, LV ejection fraction <50%, β‐blocker use at baseline, and missing accelerometer data. The final study population included 1368 participants (Figure S1). Participants provided written informed consent. The University of Texas Southwestern Institutional Review Board approved the phase 1 and phase 2 of the DHS.
Baseline Covariates
Baseline demographic characteristics, blood pressure, body mass index (BMI), medication use, prevalent risk factors and cardiovascular disease, and fasting laboratory values were assessed using standardized protocols, as previously reported. 16 Cardiac biomarkers, including high‐sensitivity cardiac troponin T and NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide), were measured using the Elecsys 2010 platform (Roche Diagnostics, Indianapolis, IN), as previously reported. 17 Insulin resistance was measured using the Homeostatic Model Assessment of Insulin Resistance, and was calculated as follows: [fasting glucose (mmol/L)×level of fasting insulin (μU/mL)]/22.5. 18 Whole body total fat percentage and whole body total lean mass were measured using dual‐energy x‐ray absorptiometry (Delphi W scanner [Hologic Inc, Bedford, MA] and Discovery software, version 12.2), as reported previously. 19
Objective Assessment of Physical Activity Levels and Sedentary Time
Study participants wore an accelerometer (Actical; Philips Respironics, Bend, OR) for 7 consecutive days on their nondominant wrist to measure sedentary time and physical activity. This method for physical activity assessment in DHS‐2 has been described previously. 20 Sedentary time was identified using a threshold of <100 counts per minute (CPM). 21 , 22 As the focus of the analysis was daytime sedentary behavior, sedentary time analysis was limited to daytime hours from 8 am to 8 pm to reduce the misclassification of sleep as sedentary time. Total minutes spent <100 CPM was divided by the number of days to assess average daily sedentary time. On the basis of prior accelerometer calibration studies, moderate physical activity was defined as 1500 to 4000 CPM and corresponds to 3.5 to 5.99 metabolic equivalent tasks (METs), whereas vigorous physical activity was defined as >4000 counts/min and corresponds to ≥6 METs. 22 , 23 , 24 , 25 Minutes spent above the threshold of 4000 CPM and between 1500 to 4000 CPM were averaged across all valid wear days to estimate the total daily duration of vigorous and moderate physical activity, respectively. Every minute above the threshold was included in the estimation of the daily moderate and vigorous physical activity levels, irrespective of the activity bout duration. The total dose of physical activity for each category (MET min/d) was calculated as the product of duration of moderate or vigorous intensity physical activity per day (in minutes) and respective mean energy expenditure rate, assigning a mean value of 4.5 METs for moderate intensity physical activity (range, 3–6 METs) and 9 METs for vigorous intensity physical activity (range, >6 METs).
Assessment of CRF Levels
CRF was estimated during a submaximal exercise test protocol. 26 Participants underwent an incremental treadmill test during which their heart rate was measured at various treadmill speeds using a Polar heart monitor. Heart rates were recorded at rest, at 2 miles per hour, and then at a third and fourth speed. These later speeds were dependent on the participant's heart rate at 2 miles per hour. If the participant's heart rate at 2 miles per hour was <100, 100 to 105, 105 to 110, 110 to 115, 115 to 120, or >120 beats per minute, the next speed was adjusted to 4, 3.5, 3.0, 2.5, 2.3, or 2.0 miles per hour, respectively. Heart rates were measured 1 minute after the participant achieved a constant speed. CRF was calculated from the estimated peak exercise oxygen consumption uptake (VO2) using Hellerstein's observation of the linear relationship between percentage maximum heart rate (MHR) and percentage peak VO2. 27 Each participant's VO2 at the speed of his/her fourth heart rate recording was calculated by a variant of the Givoni equation for metabolic energy cost: VO2=[2.3+0.32×(speed in km/h−2.5)1.65]×3.5. 28 MHR was calculated as 220 beats per minute minus participant's age. The percentage MHR was calculated for each participant as the fourth heart rate recording/MHR. Finally, Hellerstein's formula was used to convert percentage MHR into a final peak VO2 estimate: VO2/(1.41×%MHR)−42, where VO2 was calculated on the basis of speed at the fourth heart rate recording.
Assessment of Cardiac Structure and Function
Resting CMR examination included short‐axis and breath‐hold electrocardiographic‐gated cine images using a 3‐T system (Achieva; Philips Medical Systems, Best, the Netherlands). LV mass, LV end‐diastolic volume (LVEDV), and LV end‐systolic volume were calculated from short‐axis sequences by parameters that have been previously described. 29 , 30 Stroke volume (SV) was calculated as the difference between LVEDV and LV end‐systolic volume. Left atrial maximum volume was measured following the American Society of Echocardiography's guidelines, as reported previously. 31 , 32 Effective arterial elastance, a measure of the arterial load or LV afterload, was estimated as the ratio of end‐systolic pressure (0.9×systolic blood pressure) and SV. 33 LV hypertrophy was defined by increased LV mass indexed to allometric height at >97.5th percentile of the previously reported healthy subpopulation (>38.1 g/m2.7 for men and 34.1 g/m2.7 for women). 29 Chronic myocardial injury was identified by elevated levels of biomarkers of high‐sensitivity cardiac troponin T ≥6 ng/L. 12 , 13 , 34 Malignant LV hypertrophy was defined as LV hypertrophy with evidence of chronic myocardial injury. 12 , 13
Peak systolic circumferential strain was evaluated using the commercially available harmonic phase imaging software (HARP, Diagnosoft Virtue 5.04, Palo Alto, CA), as described previously. 35 A global circumferential strain curve was generated by measuring strain values in the midventricular short‐axis view from 6 midwall LV segments at various points in the cardiac cycle. The peak systolic circumferential strain, which is a representation of LV shortening in the circumferential plane, sits at the most negative point on the global circumferential strain curve. A more negative value for the peak systolic circumferential strain indicates increased LV shortening, suggesting preserved or unimpaired myocardial contractility.
Statistical Analysis
Study participants were stratified according to age‐, sex‐, and race‐specific quartiles of moderate physical activity, vigorous physical activity, and sedentary time (quartile 1, low; and quartile 4, high). Baseline clinical characteristics, cardiometabolic parameters, and CMR‐based measures of cardiac structure and function were compared across quartiles using χ2 test for categorical variables and Kruskal‐Wallis test for continuous variables. Unadjusted correlations between objectively measured moderate physical activity, vigorous physical activity, sedentary time, and CRF were assessed using Spearman correlation coefficients. The proportion of low fit participants, defined as those in the lowest quartile of the age‐, sex‐, and race‐specific CRF levels, was also compared across quartiles of moderate physical activity, vigorous physical activity, and sedentary time. Multivariable‐adjusted linear regression models were constructed to evaluate the association of sedentary time, moderate physical activity, vigorous physical activity, and CRF (exposure variables of interest) with CMR measures of cardiac structure and function (dependent variable). Separate models were constructed for each exposure variable with adjustment for the following confounders (model 1): baseline age, sex, race/ethnicity, education level, income, BMI, smoking status, systolic blood pressure, history of diabetes mellitus, history of hypertension, blood glucose levels, serum low‐density lipoprotein cholesterol, and family history of coronary artery disease. Sedentary time, moderate physical activity, vigorous physical activity, and CRF were subsequently included in the same model along with other covariates (model 2) to determine the independent associations of these measures with cardiac structure and function. Stratified analyses were performed to determine the associations of CRF and physical activity levels with measures of cardiac structure and function across sex and BMI (obese versus nonobese) based subgroups. Sensitivity analyses were also performed, replacing BMI with measures of lean body mass and fat mass, as assessed by dual‐energy x‐ray absorptiometry, among study participants in the most adjusted model.
The association of CRF and vigorous physical activity levels with presence of LV hypertrophy (LV hypertrophy with myocardial injury [malignant LV hypertrophy] versus LV hypertrophy without myocardial injury versus no LV hypertrophy with prevalent myocardial injury versus no LV hypertrophy and no myocardial injury [referent]) was assessed using multivariable logistic models with adjustment for potential confounders, as included in model 1. The statistical analysis was performed using SAS 9.4 (SAS Institute Inc, Cary, NC). The statistical tests were 2 sided, with P<0.05 considered statistically significant.
Results
Baseline Characteristics
The final study population included 1368 study participants. Baseline characteristics of the study participants, stratified by quartiles of moderate physical activity, are shown in Table 1. Participants with higher levels of moderate physical activity had a favorable cardiometabolic profile with lower burden of diabetes mellitus and central adiposity, lower CRP (C‐reactive protein) and triglyceride levels, and higher high‐density lipoprotein cholesterol and CRF levels.
Table 1.
Baseline Characteristics, Stratified by Quartiles of Moderate Physical Activity
| Characteristics | Quartile 1 (N=313) | Quartile 2 (N=350) | Quartile 3 (N=374) | Quartile 4 (N=331) | P Value |
|---|---|---|---|---|---|
| Quartiles of moderate activity (MET min/d) | |||||
| Age, y | 49.2 (9.8) | 49.2 (10.1) | 49.2 (10.6) | 49.2 (10.2) | 0.97 |
| Men, % | 39.3 | 40.9 | 40.9 | 39.9 | 0.89 |
| Race/ethnicity, % | |||||
| White | 39 | 38 | 37.1 | 38.7 | 0.88 |
| Black | 45.1 | 42 | 43 | 46.8 | 0.58 |
| Hispanic | 14.4 | 15.4 | 16.9 | 15.1 | 0.68 |
| Education, y | 12.7 (2.2) | 13.0 (2.1) | 13.0 (2.1) | 12.5 (2.5) | 0.33 |
| Smoking, % | 24.3 | 17.7 | 17.4 | 20.7 | 0.31 |
| History of hypertension, % | 42.2 | 41.7 | 39.3 | 40.8 | 0.58 |
| History of DM, % | 14.4 | 12.3 | 9.1 | 8.2 | 0.01 |
| Family history of CAD, % | 67.4 | 68.6 | 64.2 | 65 | 0.29 |
| Cardiometabolic parameters | |||||
| Systolic BP, mm Hg | 128.2 (16.9) | 130.0 (18.8) | 130.0 (18.0) | 129.8 (16.7) | 0.19 |
| hs‐CRP | 6.8 (41.2) | 4.0 (7.3) | 4.6 (10.2) | 3.3 (5.0) | 0.02 |
| LDL, mg/dL | 117.9 (32.5) | 118.2 (35.8) | 117.8 (35.7) | 119.7 (36.0) | 0.86 |
| HDL, mg/dL | 52.1 (14.6) | 53.6 (16.1) | 54.2 (15.3) | 55.8 (16.3) | <0.01 |
| Triglycerides, mg/dL | 128.9 (82.8) | 126.2 (113.4) | 121.8 (85.0) | 110.4 (71.4) | <0.01 |
| Glucose, mg/dL | 103.1 (41.1) | 101.4 (36.9) | 97.3 (24.8) | 94.9 (21.4) | 0.16 |
| Hemoglobin A1c, % | 5.8 (1.3) | 5.7 (1.2) | 5.6 (0.9) | 5.5 (0.6) | 0.36 |
| HOMA‐IR | 4.2 (4.1) | 3.6 (2.8) | 3.6 (3.5) | 3.1 (3.0) | <0.01 |
| BMI, kg/m2 | 29.4 (5.9) | 29.3 (5.2) | 29.3 (5.5) | 28.8 (5.3) | 0.10 |
| Whole body fat, % | 37.3 (8.5) | 36.8 (8.5) | 36.8 (8.7) | 35.5 (9.1) | 0.05 |
| Waist circumference, cm | 94.2 (14.6) | 93.7 (12.0) | 93.3 (12.9) | 91.7 (12.7) | <0.01 |
| Sedentary time, physical activity, and fitness parameters | |||||
| Sedentary time, min/d | 368.9 (94.1) | 317.9 (75.7) | 272.8 (75.3) | 224.7 (80.8) | <0.01 |
| Moderate physical activity, min/d | 10.4 (3.4–16.3) | 24.3 (17.3–31.6) | 42.5 (32.5–53.0) | 79.9 (61.6–103.7) | <0.01 |
| Moderate physical activity, MET min/d | 46.7 (28.7–73.1) | 109.1 (77.6–142.1) | 191.4 (146.3–238.5) | 359.4 (277.3–466.5) | <0.01 |
| Vigorous physical activity, min/d | 0.0 (0.0–0.4) | 0.3 (0.0–1.0) | 0.8 (0.3–1.9) | 1.50 (0.6–4.3) | <0.01 |
| Vigorous physical activity, MET min/d | 0.0 (0.0–3.4) | 2.6 (0.0–9.0) | 7.5 (2.3–16.9) | 13.5 (5.4–38.3) | <0.01 |
| Peak VO2, mL/kg per min | 26.5 (9.2) | 28.4 (9.6) | 29.1 (10.8) | 29.9 (10.7) | <0.01 |
| Cardiac MRI parameters | |||||
| Stroke volume, mL | 79.4 (15.9) | 80.6 (16.0) | 81.6 (16.2) | 81.5 (16.8) | 0.10 |
| LA maximum volume, mL | 60.7 (21.0) | 63.0 (21.6) | 62.2 (21.0) | 63.2 (22.5) | 0.36 |
| Peak systolic strain, s−1 | –14.4 (2.8) | –14.7 (2.6) | –14.8 (2.9) | –14.6 (2.7) | 0.43 |
| Effective arterial elastance, mm Hg/mL | 1.53 (0.3) | 1.5 (0.4) | 1.5 (0.4) | 1.5 (0.4) | 0.26 |
| LV mass indexed to BSA, g/m2 | 63.2 (15.0) | 63.6 (14.1) | 63.8 (14.3) | 65.2 (14.4) | 0.08 |
| LVEDV indexed to BSA, mL/m2 | 57.9 (10.6) | 59.0 (10.4) | 59.8 (11.1) | 61.1 (11.7) | <0.01 |
Data presented as mean (SD) for continuous variables (except physical activity parameters, which are reported as median [interquartile range]) and proportions for categorical variables. BMI indicates body mass index; BP, blood pressure; BSA, body surface area; CAD, coronary artery disease; DM, diabetes mellitus; HDL, high‐density lipoprotein; HOMA‐IR, Homeostatic Model Assessment of Insulin Resistance; hs‐CRP, high‐sensitivity C‐reactive protein; LA, left atrial; LDL, low‐density lipoprotein; LV, left ventricular; LVEDV, LV end‐diastolic volume; MET, metabolic equivalent task; MRI, magnetic resonance imaging; and VO2, oxygen consumption uptake.
Baseline characteristics of the study participants, stratified by quartiles of vigorous physical activity, are shown in Table 2. Participants with higher levels of vigorous physical activity (quartile 4) had lower anthropometric measures of adiposity (BMI and waist circumference), lower sedentary time, and a more favorable cardiometabolic risk profile with lower triglyceride levels, lower prevalence of diabetes mellitus, hypertension, and smoking, and higher high‐density lipoprotein cholesterol levels. Among measures of cardiac structure and function, participants with high levels of vigorous physical activity had higher LVEDV and a trend toward higher SV.
Table 2.
Baseline Characteristics, Stratified by Quartiles of Vigorous Physical Activity
| Characteristics | Quartile 1 (N=306) | Quartile 2 (N=369) | Quartile 3 (N=359) | Quartile 4 (N=334) | P Value |
|---|---|---|---|---|---|
| Quartiles of vigorous activity (MET min/d) | |||||
| Age, y | 48.4 (8.7) | 50.3 (11.2) | 48.6 (10.3) | 49.3 (10.1) | 0.73 |
| Men, % | 41.2 | 37.9 | 42.6 | 39.5 | 0.96 |
| Race/ethnicity, % | |||||
| White | 32.7 | 43.1 | 37.0 | 38.9 | 0.38 |
| Black | 49.0 | 39.8 | 42.6 | 46.1 | 0.73 |
| Hispanic | 15.4 | 14.4 | 17.1 | 15.3 | 0.75 |
| Education, y | 12.8 (1.8) | 12.8 (2.1) | 12.6 (2.6) | 12.9 (2.2) | 0.20 |
| Smoking, % | 29.4 | 19.2 | 18.1 | 13.9 | <0.01 |
| History of hypertension, % | 46.7 | 39.8 | 39.0 | 38.9 | 0.06 |
| History of DM, % | 15.4 | 11.9 | 8.1 | 8.7 | <0.01 |
| Family history of CAD, % | 69.6 | 67.2 | 65.5 | 62.9 | 0.06 |
| Cardiometabolic parameters | |||||
| Systolic BP, mm Hg | 131.0 (18.9) | 129.3 (17.1) | 128.8 (17.4) | 129.1 (17.3) | 0.17 |
| hs‐CRP | 6.9 (42.1) | 4.0 (5.4) | 3.5 (7.2) | 4.3 (8.4) | 0.08 |
| LDL, mg/dL | 117.2 (33.3) | 120.8 (36.6) | 119.0 (36.4) | 116.1 (33.5) | 0.40 |
| HDL, mg/dL | 53.3 (15.8) | 53.4 (14.9) | 52.8 (14.4) | 56.4 (17.2) | 0.03 |
| Triglycerides, mg/dL | 119.8 (67.5) | 125.5 (111.6) | 129.8 (92.6) | 111.0 (77.1) | <0.01 |
| Glucose, mg/dL | 102.1 (36.1) | 101.5 (38.9) | 97.1 (28.6) | 95.9 (20.5) | 0.9 |
| Hemoglobin A1c, % | 5.8 (1.2) | 5.7 (1.1) | 5.6 (1.0) | 5.5 (0.7) | 0.23 |
| HOMA‐IR | 4.0 (3.8) | 3.7 (3.2) | 3.5 (3.6) | 3.2 (3.0) | <0.01 |
| BMI, kg/m2 | 29.6 (5.8) | 29.5 (5.3) | 30.0 (5.5) | 28.7 (5.3) | <0.01 |
| Whole body fat, % | 36.6 (8.7) | 38.0 (8.2) | 36.0 (8.7) | 35.8 (9.3) | 0.06 |
| Waist circumference, cm | 95.6 (14.0) | 93.8 (12.9) | 92.5 (13.0) | 91.2 (12.0) | <0.01 |
| Sedentary time, physical activity, and fitness parameters | |||||
| Sedentary time, min | 336.1 (97.6) | 306.1 (93.2) | 276.8 (92.9) | 263.5 (87.3) | <0.01 |
| Moderate physical activity, min/d | 16.9 (9.2–31.0) | 25.6 (13.4–43.0) | 40.3 (25.6–62.7) | 53.9 (32.4–84.1) | <0.01 |
| Moderate physical activity, MET min/d | 75.9 (41.3–139.5) | 115.9 (60.2–193.5) | 181.1 (115.3–282.2) | 242.6 (145.9–378.6) | <0.01 |
| Vigorous physical activity, min/d | 0.0 (0.0–0.0) | 0.3 (0.0–0.4) | 1.0 (0.6–1.4) | 3.3 (1.9–7.3) | <0.01 |
| Vigorous physical activity, MET min/d | 0.0 (0.0–0.0) | 2.3 (0.0–3.9) | 9.0 (5.1–12.9) | 29.6 (16.7–65.3) | <0.01 |
| Peak VO2, mL/kg per min | 30.0 (9.2) | 27.8 (10.0) | 28.6 (9.9) | 30.6 (11.1) | <0.01 |
| Cardiac MRI parameters | |||||
| Stroke volume, mL | 80.5 (14.6) | 79.3 (16.4) | 80.6 (16.8) | 82.9 (16.7) | 0.06 |
| LA maximum volume, mL | 63.8 (21.2) | 60.9 (20.6) | 61.2 (21.9) | 63.8 (22.3) | 0.77 |
| Peak systolic strain, s−1 | –14.5 (2.7) | –14.5 (2.6) | –14.7 (2.9) | –14.8 (2.8) | 0.11 |
| Effective arterial elastance, mm Hg/mL | 1.5 (0.3) | 1.6 (0.4) | 1.5 (0.4) | 1.5 (0.4) | <0.01 |
| LV mass indexed to BSA, g/m2 | 64.6 (15.7) | 62.5 (13.0) | 63.6 (13.7) | 65.5 (15.4) | 0.28 |
| LVEDV indexed to BSA, mL/m2 | 58.5 (10.2) | 58.1 (11.1) | 59.4 (10.7) | 61.9 (11.5) | <0.01 |
Data presented as mean (SD) for continuous variables (except physical activity parameters, which are reported as median [interquartile range]) and proportions for categorical variables. BMI indicates body mass index; BP, blood pressure; BSA, body surface area; CAD, coronary artery disease; DM, diabetes mellitus; HDL, high‐density lipoprotein; HOMA‐IR, Homeostatic Model Assessment of Insulin Resistance; hs‐CRP, high‐sensitivity C‐reactive protein; LA, left atrial; LDL, low‐density lipoprotein; LV, left ventricular; LVEDV, LV end‐diastolic volume; MET, metabolic equivalent task; MRI, magnetic resonance imaging; and VO2, oxygen consumption uptake.
Baseline characteristics of study participants across increasing sedentary time quartiles are shown in Table S1. Compared with participants with low sedentary time (quartile 1), those with higher sedentary time levels had higher education levels. Furthermore, participants with higher sedentary time levels had less favorable cardiometabolic risk profile with higher triglyceride levels and insulin resistance (assessed by Homeostatic Model Assessment of Insulin Resistance).
Association Between Physical Activity, Sedentary Time, and CRF Levels
A modest correlation was observed between physical activity levels and CRF (correlation coefficient for moderate physical activity and CRF, 0.17 [P<0.001]; and correlation coefficient for vigorous physical activity and CRF, 0.25 [P<0.001]). Moderate physical activity levels were also correlated with vigorous physical activity levels (correlation coefficient, 0.57; P<0.0001) and sedentary time duration (correlation coefficient, −0.61; P<0.0001). In contrast, sedentary time duration had a modest inverse correlation with vigorous physical activity levels (correlation coefficient, −0.28; P<0.0001) and no association with CRF levels (correlation coefficient, −0.02; P=0.49).
The Figure shows that proportion of low fit participants (lowest age‐, sex‐, and race‐specific quartile of CRF) decreased across increasing moderate (31% in quartile 1 to 19% in quartile 4) and vigorous physical activity (27% in quartile 1 to 19% in quartile 4) categories; conversely, proportion of low fit participants did not differ across increasing quartiles of sedentary time (20% in quartile 1 to 23% in quartile 4).
Figure 1. Proportion of participants with low fitness across data‐derived categories of moderate physical activity (top panel), vigorous physical activity (middle panel), and sedentary time (bottom panel).
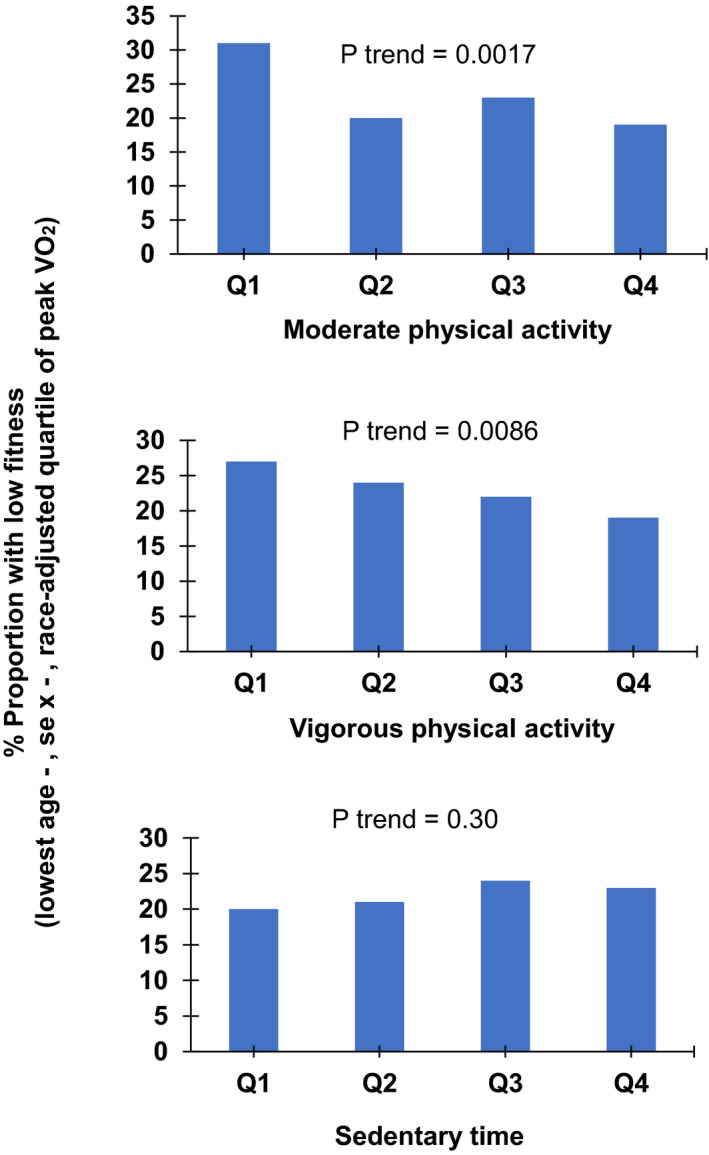
Low fit participants are identified as those in the lowest age‐, sex‐, and race‐adjusted quartile of peak oxygen consumption uptake (VO2). Q indicates quartile.
Association of CRF, Physical Activity, and Sedentary Time With Cardiac Structure and Function
After adjustment for baseline demographic characteristics and cardiovascular risk factors (model 1), CRF was positively associated with larger LVEDV and left atrial maximum volume and higher LV mass (Table 3). Among measures of cardiac performance, CRF was positively associated with higher SV, better LV contractility (more negative strain), and lower effective arterial elastance.
Table 3.
Association Between Sedentary Time, Moderate Physical Activity, Vigorous Physical Activity, and CRF With Measures of Cardiac Structure and Function After Adjustment for Baseline Confounders
| CMR Outcome | Cardiorespiratory Fitness | Vigorous Physical Activity | Moderate Physical Activity | Sedentary Time | ||||
|---|---|---|---|---|---|---|---|---|
| Standardized ß* (95% CI) | P Value | Standardized ß* (95% CI) | P Value | Standardized ß* (95% CI) | P Value | Standardized ß (95% CI) | P Value | |
| Stroke volume | 0.27 (0.22 to 0.33) | <0.01 | 0.12 (0.06 to 0.16) | <0.01 | 0.06 (0.01 to 0.11) | 0.02 | –0.04 (–0.09 to 0.01) | 0.11 |
| LA maximum volume | 0.18 (0.12 to 0.25) | <0.01 | 0.03 (–0.03 to 0.08) | 0.36 | 0.03 (–0.03 to 0.09) | 0.27 | –0.04 (–0.09 to 0.02) | 0.23 |
| Peak systolic strain | –0.16 (–0.22 to –0.10) | <0.01 | –0.03 (–0.08 to 0.03) | 0.37 | 0.002 (–0.05 to 0.06) | 0.94 | 0.01 (–0.05 to 0.06) | 0.80 |
| Effective arterial elastance | –0.22 (–0.27 to –0.17) | <0.01 | –0.09 (–0.13 to –0.05) | <0.01 | –0.04 (–0.08 to 0.01) | 0.09 | 0.03 (–0.02 to 0.07) | 0.22 |
| LV mass indexed to BSA | 0.15 (0.11 to 0.20) | <0.01 | 0.10 (0.06 to 0.13) | <0.01 | 0.02 (–0.02 to 0.06) | 0.32 | –0.004 (–0.04 to 0.03) | 0.84 |
| LVEDV indexed to BSA | 0.24 (0.19 to 0.15) | <0.01 | 0.11 (0.06 to 0.15) | <0.01 | 0.06 (0.01 to 0.11) | 0.01 | –0.04 (–0.08 to 0.01) | 0.13 |
Model was adjusted for baseline age, sex, race/ethnicity, education level, income, and traditional cardiovascular risk factors (body mass index, smoking status, systolic blood pressure, history of diabetes mellitus, history of hypertension, blood glucose levels, serum low‐density lipoprotein cholesterol, and family history of coronary artery disease). BSA indicates body surface area; CMR, cardiac magnetic resonance imaging; CRF, cardiorespiratory fitness; LA, left atrial; LV, left ventricular; and LVEDV, LV end‐diastolic volume.
Standardized ß estimate for the association between exposure of interest (physical activity, sedentary time, and CRF) and each CMR outcome represents the number of SDs the outcome will change per 1‐SD higher exposure variable, keeping other covariates fixed.
Among physical activity levels, vigorous physical activity was positively associated with larger LVEDV, higher LV mass, higher SV, and lower effective arterial elastance. Amount of vigorous physical activity was not associated with left atrial size and LV contractility in adjusted analysis (Table 3). Moderate physical activity level was significantly associated with higher SV and larger LVEDV but not with other cardiac parameters after adjustment for baseline confounders. In contrast, sedentary time was not associated with measures of cardiac structure and function in adjusted analysis (Table 3).
The significant associations between CRF, vigorous physical activity, and measures of LV structure and function were not attenuated when all physical activity and CRF parameters were included in the same model (Table 4). In contrast, the associations of moderate physical activity with SV and LVDEV were attenuated and no longer significant after additional adjustment for CRF, vigorous physical activity, and sedentary time in the most adjusted model. No significant collinearity was observed between the CRF and physical activity parameters in the most adjusted model (variance inflation factor <2 for all). Similar patterns of association were observed in sensitivity analysis, adjusting for measures of body composition (lean body mass and fat mass) instead of BMI (Table S2). In addition, the association between CRF and parameters of LV structure and function was not attenuated in sex‐ and obesity‐stratified models (Table S3 and S4). However, the association of vigorous physical activity with LV structure and function was attenuated in women and in the obese subgroup.
Table 4.
Association Between Sedentary Time, Moderate Physical Activity, Vigorous Physical Activity, and CRF With Measures of Cardiac Structure and Function, Independent of Baseline Confounders and Each Other
| CMR Outcome | Cardiorespiratory Fitness | Vigorous Physical Activity | Moderate Physical Activity | Sedentary Time | ||||
|---|---|---|---|---|---|---|---|---|
| Standardized ß* (95% CI) | P Value | Standardized ß* (95% CI) | P Value | Standardized ß* (95% CI) | P Value | Standardized ß (95% CI) | P Value | |
| Stroke volume | 0.26 (0.21 to 0.32) | <0.01 | 0.09 (0.04 to 0.14) | 0.0004 | 0.003 (–0.06 to 0.06) | 0.93 | –0.02 (–0.07 to 0.04) | 0.54 |
| LA maximum volume | 0.18 (0.11 to 0.25) | <0.01 | 0.006 (–0.05 to 0.06) | 0.85 | 0.008 (–0.06 to 0.08) | 0.83 | –0.02 (–0.09 to 0.05) | 0.51 |
| Peak systolic strain | –0.16 (–0.22 to –0.10) | <0.01 | –0.02 (–0.08 to 0.04) | 0.54 | 0.03 (–0.04 to 0.09) | 0.47 | 0.01 (–0.05 to 0.08) | 0.72 |
| Effective arterial elastance | –0.21 (–0.26 to –0.16) | <0.01 | –0.07 (–0.12 to –0.03) | 0.002 | 0.01 (–0.05 to 0.06) | 0.83 | 0.01 (–0.04 to 0.06) | 0.60 |
| LV mass indexed to BSA | 0.14 (0.10 to 0.19) | <0.01 | 0.09 (0.05 to 0.13) | <0.01 | –0.01 (–0.06 to 0.03) | 0.58 | 0.004 (–0.04 to 0.05) | 0.86 |
| LVEDV indexed to BSA | 0.23 (0.18 to 0.29) | <0.01 | 0.08 (0.03 to 0.13) | 0.001 | 0.01 (–0.04 to 0.07) | 0.65 | –0.01 (–0.06 to 0.04) | 0.70 |
Model was adjusted for baseline age, sex, race/ethnicity, education level, income, traditional cardiovascular risk factors (body mass index, smoking status, systolic blood pressure, history of diabetes mellitus, history of hypertension, blood glucose levels, serum low‐density lipoprotein cholesterol, and family history of coronary artery disease), as well as levels of moderate physical activity, vigorous physical activity, CRF, and sedentary time levels (all in the same model). BSA indicates body surface area; CMR, cardiac magnetic resonance imaging; CRF, cardiorespiratory fitness; LA, left atrial; LV, left ventricular; and LVEDV, LV end‐diastolic volume.
Standardized ß estimate for the association between exposure of interest (physical activity, sedentary time, and CRF) and each CMR outcome represents the number of SDs the outcome will change per 1‐SD higher exposure variable, keeping other covariates fixed.
Association of Vigorous Physical Activity and CRF With Malignant LV Hypertrophy
Because of the observed significant associations of higher CRF and vigorous physical activity with higher LV mass and larger LVEDV, we evaluated the associations of CRF with presence of malignant LV hypertrophy, as defined by LV hypertrophy and prevalent chronic myocardial injury. Malignant LV hypertrophy was observed in 8% (n=106) of individuals, whereas 7% (n=97) had LV hypertrophy without chronic myocardial injury. In multivariable logistic regression models adjusted for demographic characteristics and cardiovascular risk factors, CRF was associated with higher likelihood of LV hypertrophy without chronic myocardial injury but not with malignant LV hypertrophy. In contrast, vigorous physical activity levels were associated with higher likelihood of both malignant LV hypertrophy as well as LV hypertrophy without chronic myocardial injury (Table 5).
Table 5.
Association Between Cardiorespiratory Fitness, Vigorous Physical Activity, and Likelihood of Various LV Hypertrophy Phenotypes
| Variable | Cardiorespiratory Fitness | Vigorous Physical Activity | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| No LVH/no myocardial injury | Reference | Reference | Reference | Reference |
| No LVH/+myocardial injury | 0.96 (0.80–1.15) | 0.66 | 1.04 (0.88–1.24) | 0.64 |
| LVH/no myocardial injury | 1.44 (1.10–1.91) | <0.01 | 1.35 (1.09–1.68) | <0.01 |
| LVH/+myocardial injury (malignant LVH) | 1.33 (0.96–1.85) | 0.08 | 1.50 (1.19–1.90) | <0.01 |
Separate multivariable‐adjusted logistic regression models were constructed for primary exposure variables of cardiorespiratory fitness and vigorous physical activity with the 4‐level outcome of LV hypertrophy/myocardial injury (no LVH/no myocardial injury [reference], no LVH/+myocardial injury, LVH/no myocardial injury, and LVH/+myocardial injury). Models were adjusted for same covariates as in model 1 (see Table 3). LV indicates left ventricular; LVH, LV hypertrophy; and OR, odds ratio.
Discussion
We observed several important findings in our study. First, objective measures of moderate and vigorous physical activity but not sedentary time were significantly associated with CRF levels. Second, CRF was positively associated with more favorable LV structure and function parameters, with better LV contractility, higher SV, lower arterial elastance, and LV hypertrophy without myocardial injury, independent of traditional cardiovascular risk factors. Vigorous physical activity was also positively associated with higher measures of SV and lower effective arterial elastance, and greater likelihood of both LV hypertrophy without myocardial injury as well as malignant LV hypertrophy. Finally, moderate physical activity levels and sedentary time were not associated with LV structure and function in the most adjusted analysis. Taken together, these findings highlight the independent contributions of objective measures of CRF, physical activity, and sedentary time toward cardiac structure and function.
Higher levels of CRF in midlife are strongly associated with lower risk of HF in older age, independent of other prevalent or antecedent risk factors. 7 , 36 , 37 , 38 In a study from the CCLS (Cooper Center Longitudinal Study), each 1‐MET higher level of fitness was associated with 17% lower risk of HF. 38 Furthermore, low CRF also explained up to 40% of the high BMI associated risk of HF. 36 Similar to CRF, higher levels of physical activity have also been associated with lower risk of HF. 39 , 40 A potential mechanism by which high CRF and physical activity may be associated with lower risk of HF is through their favorable effects on cardiac structure and function, 41 , 42 which are well established in the literature as physiological consequences of long‐term aerobic exercise and physical activity. 43 Furthermore, cardiac parameters, such as higher SV, better LV contractility (more negative strain), and lower arterial stiffness, are associated with lower risk of HF. 44 , 45 , 46 , 47 In the present study, we observed a significant association between higher CRF levels and these favorable measures of cardiac structure and function, and physiologic LV hypertrophy. These findings suggest that high fitness–associated lower risk of HF may be in part related to its favorable effects on cardiac structure and function.
Similar to CRF, previous studies have also demonstrated that higher levels of physical activity and greater lifetime exercise levels have been associated with favorable cardiac structure and function. 48 , 49 , 50 However, these studies were limited by use of self‐reported physical activity levels and the lack of distinction between moderate versus vigorous intensity physical activity. Our study adds to the existing literature by evaluating the independent association of objectively measured moderate and vigorous physical activity levels with LV structure and function. We observed that vigorous but not moderate physical activity levels were associated with measures of LV structure and function. Taken together, these findings suggest that CRF and vigorous physical activity are important determinants of cardiac structure and function.
In addition to low CRF and physical activity levels, higher levels of sedentary time have also been associated with higher risk of HF and greater burden of abnormalities in LV structure. 8 , 15 However, these studies were limited by use of self‐reported measures of physical activity and sedentary time, which could have led to recall bias and greater potential for confounding from overall health status and comorbidity burden. In the present study, we observed that objective measures of sedentary time were not associated with measures of LV structure and function after adjustment for traditional HF risk factors. This is in contrast to prior observations from the DHS cohort, where sedentary time levels were significantly associated with higher levels of subclinical atherosclerosis. 21 Taken together, these findings suggest that although sedentary time is an important risk factor for atherosclerotic cardiovascular disease, it may not contribute to abnormalities in cardiac structure and function, and perhaps risk of HF, independent of other risk factors. To our knowledge, this is the first study to evaluate the associations of objective measures of physical activity and sedentary time with measures of LV structure and function.
Our study findings have important clinical implications. Findings from our study suggest that low CRF is independently associated with abnormalities in LV structure and function and may contribute to risk of HF. Given the growing burden of HF in the community, these findings highlight the importance of assessing CRF to identify low fit individuals who may have higher burden of abnormal cardiac structure and function and potentially higher risk of HF. Furthermore, these findings also suggest that lifestyle interventions that target improvements in CRF levels through exercise and weight loss may be needed to modify long‐term risk of HF. 7 , 51 Along these lines, we also observed that vigorous intensity, but not moderate intensity, physical activity is associated with measures of cardiac structure and function. Future studies are needed using high intensity exercise training to determine if lifestyle interventions aimed at increasing vigorous intensity physical activity levels and CRF over time will modify LV structure and function.
The key strengths of our study include the large sample size and the objective measurements of sedentary time and physical activity levels, and magnetic resonance imaging–based assessment of cardiac structure and function in the DHS participants. Several limitations to our study are also noteworthy. First, because of the observational nature of our study, our observations are prone to residual and unmeasured confounding. Second, because of the cross‐sectional nature of our analysis, our study findings do not establish a causal association between CRF, physical activity levels, and measures of cardiac structure and function. Also, we could not evaluate if changes in CRF or physical activity levels were associated with changes in cardiac structure and function. However, prior studies have demonstrated that longitudinal changes in CRF are significantly associated with cardiac structure and function, highlighting the potentially modifiable nature of this association. 41 Third, our study was limited to participants who underwent complete submaximal CRF testing and had available CRF data. Thus, our study findings may not be generalizable to individuals who may not meet these inclusion criteria. Fourth, sedentary time and physical activity were exclusively measured over a 7‐day period, and thus may not reflect the full extent of participants' long‐term sedentary behaviors and physical activity. Fifth, as per the study protocol, sedentary time was assessed between 8 am and 8 pm to avoid misclassification of sleep duration as sedentary time. Thus, it is plausible that the overall sedentary duration may be underestimated. However, inclusion of sleep duration into sedentary duration may confound the analysis as sleep is considered biologically distinct from daytime sedentary behavior.
In conclusion, among DHS‐2 participants, vigorous physical activity and CRF are significantly associated with cardiac structure and function parameters, independent of other risk factors. Future prospective studies are needed to determine if interventions aimed at increasing vigorous physical activity and CRF levels may favorably modify cardiac structure and function.
Sources of Funding
Dr Patel was supported by the National Heart, Lung, and Blood Institute T32 postdoctoral training grant (5T32HL125247‐03). Dr Pandey is funded by the Texas Health Resources Clinical Scholarship, Gilead Sciences Research Scholar Program, investigator initiated research grant from Applied Therapeutics, and the National Institute of Aging Grants for Early Medical/Surgical Specialists' Transition to Aging Research (1R03AG067960‐01). The DHS (Dallas Heart Study) was funded by the Donald W. Reynolds Foundation and was partially supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award UL1TR001105. This work was also supported by the American Heart Association Strategically Focused Research Grant (14SFRN20740000).
Disclosures
Dr Pandey has served on the advisory board of Roche Diagnostics. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S4
Figure S1
Acknowledgments
We would like to thank the DHS (Dallas Heart Study) and staff for their contribution to the study.
(J Am Heart Assoc.2021;10:e015601. DOI: 10.1161/JAHA.119.015601.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.119.015601
For Sources of Funding and Disclosures, see page 10.
References
- 1. Khera R, Kondamudi N, Zhong L, Vaduganathan M, Parker J, Das SR, Grodin JL, Halm EA, Berry JD, Pandey A. Temporal trends in heart failure incidence among Medicare beneficiaries across risk factor strata, 2011 to 2016. JAMA Netw Open. 2020;3:e2022190. DOI: 10.1001/jamanetworkopen.2020.22190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rahman I, Bellavia A, Wolk A, Orsini N. Physical activity and heart failure risk in a prospective study of men. JACC Heart Fail. 2015;3:681–687. [DOI] [PubMed] [Google Scholar]
- 3. Andersen K, Mariosa D, Adami HO, Held C, Ingelsson E, Lagerros YT, Nyrén O, Ye W, Bellocco R, Sundström J. Dose‐response relationship of total and leisure time physical activity to risk of heart failure: a prospective cohort study. Circ Heart Fail. 2014;7:701–708. DOI: 10.1161/CIRCHEARTFAILURE.113.001010 [DOI] [PubMed] [Google Scholar]
- 4. Khan H, Kunutsor S, Rauramaa R, Savonen K, Kalogeropoulos AP, Georgiopoulou VV, Butler J, Laukkanen JA. Cardiorespiratory fitness and risk of heart failure: a population‐based follow‐up study. Eur J Heart Fail. 2014;16:180–188.DOI: 10.1111/ejhf.37 [DOI] [PubMed] [Google Scholar]
- 5. Myers J, Kokkinos P, Chan K, Dandekar E, Yilmaz B, Nagare A, Faselis C, Soofi, M, Soofi M. Cardiorespiratory fitness and reclassification of risk for incidence of heart failure: the Veterans Exercise Testing Study. Circ Heart Fail. 2017;10:e003780. DOI: 10.1161/CIRCHEARTFAILURE.116.003780 [DOI] [PubMed] [Google Scholar]
- 6. Kupsky DF, Ahmed AM, Sakr S, Qureshi WT, Brawner CA, Blaha MJ, Ehrman JK, Keteyian SJ, Al‐Mallah MH. Cardiorespiratory fitness and incident heart failure: the Henry Ford Exercise Testing (FIT) Project. Am Heart J. 2017;185:35–42.DOI: 10.1016/j.ahj.2016.12.006 [DOI] [PubMed] [Google Scholar]
- 7. Pandey A, Patel KV, Bahnson JL, Gaussoin SA, Martin CK, Balasubramanyam A, Johnson KC, McGuire DK, Bertoni AG, Kitzman D, et al. Association of intensive lifestyle intervention, fitness, and body mass index with risk of heart failure in overweight or obese adults with type 2 diabetes mellitus: an analysis from the look ahead trial. Circulation. 2020;141:1295–1306.DOI: 10.1161/CIRCULATIONAHA.119.044865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Young DR, Reynolds K, Sidell M, Brar S, Ghai NR, Sternfeld B, Jacobsen SJ, Slezak JM, Caan B, Quinn VP. Effects of physical activity and sedentary time on the risk of heart failure. Circ Heart Fail. 2014;7:21–27.DOI: 10.1161/CIRCHEARTFAILURE.113.000529 [DOI] [PubMed] [Google Scholar]
- 9. Russo C, Jin Z, Elkind MS, Rundek T, Homma S, Sacco RL, Di Tullio MR. Prevalence and prognostic value of subclinical left ventricular systolic dysfunction by global longitudinal strain in a community‐based cohort. Eur J Heart Fail. 2014;16:1301–1309.DOI: 10.1002/ejhf.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC Jr, Jacobsen SJ, Rodeheffer RJ. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA. 2011;306:856–863.DOI: 10.1001/jama.2011.1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lam CSP, Lyass A, Kraigher‐Krainer E, Massaro JM, Lee DS, Ho JE, Levy D, Redfield MM, Pieske BM, Benjamin EJ, et al. Cardiac dysfunction and noncardiac dysfunction as precursors of heart failure with reduced and preserved ejection fraction in the community. Circulation. 2011;124:24–30.DOI: 10.1161/CIRCULATIONAHA.110.979203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pandey A, Keshvani N, Ayers C, Correa A, Drazner MH, Lewis A, Rodriguez CJ, Hall ME, Fox ER, Mentz RJ, et al. Association of cardiac injury and malignant left ventricular hypertrophy with risk of heart failure in African Americans: the Jackson Heart Study. JAMA Cardiol. 2019;4:51–58.DOI: 10.1001/jamacardio.2018.4300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lewis AA, Ayers CR, Selvin E, Neeland I, Ballantyne CM, Nambi V, Pandey A, Powell‐Wiley TM, Drazner MH, Carnethon MR, et al. Racial differences in malignant left ventricular hypertrophy and incidence of heart failure: a multicohort study. Circulation. 2020;141:957–967.DOI: 10.1161/CIRCULATIONAHA.119.043628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pluim BM, Zwinderman AH, van der Laarse A, van der Wall EE. The athlete's heart: a meta‐analysis of cardiac structure and function. Circulation. 2000;101:336–344.DOI: 10.1161/01.CIR.101.3.336 [DOI] [PubMed] [Google Scholar]
- 15. Gibbs BB, Reis JP, Schelbert EB, Craft LL, Sidney S, Lima J, Lewis CE. Sedentary screen time and left ventricular structure and function: the CARDIA study. Med Sci Sports Exerc. 2014;46:276–283.DOI: 10.1249/MSS.0b013e3182a4df33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Victor RG, Haley RW, Willett DL, Peshock RM, Vaeth PC, Leonard D, Basit M, Cooper RS, Iannacchione VG, Visscher WA, et al. The Dallas Heart Study: a population‐based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol. 2004;93:1473–1480.DOI: 10.1016/j.amjcard.2004.02.058 [DOI] [PubMed] [Google Scholar]
- 17. de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, Hashim I, Berry JD, Das SR, Morrow DA, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–2512.DOI: 10.1001/jama.2010.1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 19. Neeland IJ, Gupta S, Ayers CR, Turer AT, Rame JE, Das SR, Berry JD, Khera A, McGuire DK, Vega GL, et al. Relation of regional fat distribution to left ventricular structure and function. Circ Cardiovasc Imaging. 2013;6:800–807.DOI: 10.1161/CIRCIMAGING.113.000532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lakoski SG, Kozlitina J. Ethnic differences in physical activity and metabolic risk: the Dallas Heart Study. Med Sci Sports Exerc. 2014;46:1124–1132.DOI: 10.1249/MSS.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 21. Kulinski JP, Kozlitina J, Berry JD, de Lemos JA, Khera A. Association between sedentary time and coronary artery calcium. JACC Cardiovasc Imaging. 2016;9:1470–1472.DOI: 10.1016/j.jcmg.2016.01.013 [DOI] [PubMed] [Google Scholar]
- 22. Wong SL, Colley R, Connor Gorber S, Tremblay M. Actical accelerometer sedentary activity thresholds for adults. J Phys Act Health. 2011;8:587–591.DOI: 10.1123/jpah.8.4.587 [DOI] [PubMed] [Google Scholar]
- 23. Colley RC, Tremblay MS. Moderate and vigorous physical activity intensity cut‐points for the Actical accelerometer. J Sports Sci. 2011;29:783–789.DOI: 10.1080/02640414.2011.557744 [DOI] [PubMed] [Google Scholar]
- 24. Hooker SP, Feeney A, Hutto B, Pfeiffer KA, McIver K, Heil DP, Vena JE, Lamonte MJ, Blair SN. Validation of the Actical activity monitor in middle‐aged and older adults. J Phys Act Health. 2011;8:372–381.DOI: 10.1123/jpah.8.3.372 [DOI] [PubMed] [Google Scholar]
- 25. Heil DP. Predicting activity energy expenditure using the Actical activity monitor. Res Q Exerc Sport. 2006;77:64–80.DOI: 10.1080/02701367.2006.10599333 [DOI] [PubMed] [Google Scholar]
- 26. Pandey A, Park BD, Ayers C, Das SR, Lakoski S, Matulevicius S, de Lemos JA, Berry JD. Determinants of racial/ethnic differences in cardiorespiratory fitness (from the Dallas Heart Study). Am J Cardiol. 2016;118:499–503.DOI: 10.1016/j.amjcard.2016.05.043 [DOI] [PubMed] [Google Scholar]
- 27. Naughton TP, Hellerstien HK, Mohler IC. Exercise Testing and Exercise Training in Coronary Heart Disease. New York, London: Academic Press; 1973. [Google Scholar]
- 28. Givoni B, Goldman RF. Predicting metabolic energy cost. J Appl Physiol. 1971;30:429–433.DOI: 10.1152/jappl.1971.30.3.429 [DOI] [PubMed] [Google Scholar]
- 29. Garg S, de Lemos JA, Matulevicius SA, Ayers C, Pandey A, Neeland IJ, Berry JD, McColl R, Maroules C, Peshock RM, et al. Association of concentric left ventricular hypertrophy with subsequent change in left ventricular end‐diastolic volume: the Dallas Heart Study. Circ Heart Fail. 2017;10:e003959. DOI: 10.1161/CIRCHEARTFAILURE.117.003959 [DOI] [PubMed] [Google Scholar]
- 30. Wilner B, Garg S, Ayers CR, Maroules CD, McColl R, Matulevicius SA, de Lemos JA, Drazner MH, Peshock R, Neeland IJ. Dynamic relation of changes in weight and indices of fat distribution with cardiac structure and function: the Dallas Heart Study. J Am Heart Assoc. 2017;6:e005897. DOI: 10.1161/JAHA.117.005897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–270.DOI: 10.1093/ehjci/jev014 [DOI] [PubMed] [Google Scholar]
- 32. Oliver W, Matthews G, Ayers CR, Garg S, Gupta S, Neeland IJ, Drazner MH, Berry JD, Matulevicius S, de Lemos JA. Factors associated with left atrial remodeling in the general population. Circ Cardiovasc Imaging. 2017;10:e005047. DOI: 10.1161/CIRCIMAGING.116.005047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Coutinho T, Borlaug BA, Pellikka PA, Turner ST, Kullo IJ. Sex differences in arterial stiffness and ventricular‐arterial interactions. J Am Coll Cardiol. 2013;61:96–103.DOI: 10.1016/j.jacc.2012.08.997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pandey A, Patel KV, Vongpatanasin W, Ayers C, Berry JD, Mentz RJ, Blaha MJ, McEvoy JW, Muntner P, Vaduganathan M, et al. Incorporation of biomarkers into risk assessment for allocation of antihypertensive medication according to the 2017 ACC/AHA high blood pressure guideline: a pooled cohort analysis. Circulation. 2019;140:2076–2088.DOI: 10.1161/CIRCULATIONAHA.119.043337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pandey A, Park B, Martens S, Ayers C, Neeland IJ, Haykowsky MJ, Nelson MD, Sarma S, Berry JD. Relationship of cardiorespiratory fitness and adiposity with left ventricular strain in middle‐age adults (from the Dallas Heart Study). Am J Cardiol. 2017;120:1405–1409.DOI: 10.1016/j.amjcard.2017.07.031 [DOI] [PubMed] [Google Scholar]
- 36. Pandey A, Cornwell WK, Willis B, Neeland IJ, Gao A, Leonard D, DeFina L, Berry JD. Body mass index and cardiorespiratory fitness in mid‐life and risk of heart failure hospitalization in older age: findings from the Cooper Center Longitudinal Study. JACC Heart Fail. 2017;5:367–374.DOI: 10.1016/j.jchf.2016.12.021 [DOI] [PubMed] [Google Scholar]
- 37. Berry JD, Pandey A, Gao A, Leonard D, Farzaneh‐Far R, Ayers C, DeFina L, Willis B. Physical fitness and risk for heart failure and coronary artery disease. Circ Heart Fail. 2013;6:627–634.DOI: 10.1161/CIRCHEARTFAILURE.112.000054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pandey A, Patel M, Gao A, Willis BL, Das SR, Leonard D, Drazner MH, de Lemos JA, DeFina L, Berry JD. Changes in mid‐life fitness predicts heart failure risk at a later age independent of interval development of cardiac and noncardiac risk factors: the Cooper Center Longitudinal Study. Am Heart J. 2015;169:290–297.e1.DOI: 10.1016/j.ahj.2014.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pandey A, Garg S, Khunger M, Darden D, Ayers C, Kumbhani DJ, Mayo HG, de Lemos JA, Berry JD. Dose‐response relationship between physical activity and risk of heart failure: a meta‐analysis. Circulation. 2015;132:1786–1794.DOI: 10.1161/CIRCULATIONAHA.115.015853 [DOI] [PubMed] [Google Scholar]
- 40. Pandey A, LaMonte M, Klein L, Ayers C, Psaty BM, Eaton CB, Allen NB, de Lemos JA, Carnethon M, Greenland P, et al. Relationship between physical activity, body mass index, and risk of heart failure. J Am Coll Cardiol. 2017;69:1129–1142.DOI: 10.1016/j.jacc.2016.11.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pandey A, Allen NB, Ayers C, Reis JP, Moreira HT, Sidney S, Rana JS, Jacobs DR, Chow LS, de Lemos JA, et al. Fitness in young adulthood and long‐term cardiac structure and function: the CARDIA study. JACC Heart Fail. 2017;5:347–355.DOI: 10.1016/j.jchf.2016.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pandey A, Patel KV, Vaduganathan M, Sarma S, Haykowsky MJ, Berry JD, Lavie CJ. Physical activity, fitness, and obesity in heart failure with preserved ejection fraction. JACC Heart Fail. 2018;6:975–982. [DOI] [PubMed] [Google Scholar]
- 43. Lavie CJ, Arena R, Swift DL, Johannsen NM, Sui X, Lee D‐C, Earnest CP, Church TS, O’Keefe JH, Milani RV, et al. Exercise and the cardiovascular system: clinical science and cardiovascular outcomes. Circ Res. 2015;117:207–219.DOI: 10.1161/CIRCRESAHA.117.305205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Choi E‐Y, Rosen BD, Fernandes VRS, Yan RT, Yoneyama K, Donekal S, Opdahl A, Almeida ALC, Wu CO, Gomes AS, et al. Prognostic value of myocardial circumferential strain for incident heart failure and cardiovascular events in asymptomatic individuals: the Multi‐Ethnic Study of Atherosclerosis. Eur Heart J. 2013;34:2354–2361.DOI: 10.1093/eurheartj/eht133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. De Marco M, Gerdts E, Mancusi C, Roman MJ, Lønnebakken MT, Lee ET, Howard BV, Devereux RB, de Simone G. Influence of left ventricular stroke volume on incident heart failure in a population with preserved ejection fraction (from the Strong Heart Study). Am J Cardiol. 2017;119:1047–1052.DOI: 10.1016/j.amjcard.2016.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pandey A, Khan H, Newman AB, Lakatta EG, Forman DE, Butler J, Berry JD. Arterial stiffness and risk of overall heart failure, heart failure with preserved ejection fraction, and heart failure with reduced ejection fraction: the Health ABC Study (Health, Aging, and Body Composition). Hypertension. 2017;69:267–274.DOI: 10.1161/HYPERTENSIONAHA.116.08327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tsao CW, Lyass A, Larson MG, Levy D, Hamburg NM, Vita JA, Benjamin EJ, Mitchell GF, Vasan RS. Relation of central arterial stiffness to incident heart failure in the community. J Am Heart Assoc. 2015;4:e002189. DOI: 10.1161/JAHA.115.002189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bhella PS, Hastings JL, Fujimoto N, Shibata S, Carrick‐Ranson G, Palmer MD, Boyd KN, Adams‐Huet B, Levine BD. Impact of lifelong exercise "dose" on left ventricular compliance and distensibility. J Am Coll Cardiol. 2014;64:1257–1266.DOI: 10.1016/j.jacc.2014.03.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dawes TJ, Corden B, Cotter S, de Marvao A, Walsh R, Ware JS, Cook SA, O'Regan DP. Moderate physical activity in healthy adults is associated with cardiac remodeling. Circ Cardiovasc Imaging. 2016;9:e004712. DOI: 10.1161/CIRCIMAGING.116.004712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Turkbey EB, Jorgensen NW, Johnson WC, Bertoni AG, Polak JF, Diez Roux AV, Tracy RP, Lima JA, Bluemke DA. Physical activity and physiological cardiac remodelling in a community setting: the Multi‐Ethnic Study of Atherosclerosis (MESA). Heart. 2010;96:42–48.DOI: 10.1136/hrt.2009.178426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Patel KV, Bahnson JL, Gaussoin SA, Johnson KC, Pi‐Sunyer X, White U, Olson KL, Bertoni AG, Kitzman DW, Berry JD, et al. Association of baseline and longitudinal changes in body composition measures with risk of heart failure and myocardial infarction in type 2 diabetes. Circulation. 2020;142:2420–2430. DOI: 10.1161/circulationaha.120.050941. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4
Figure S1


