Figure 3. Examples of material systems for in vivo delivery of genome-editing machinery.
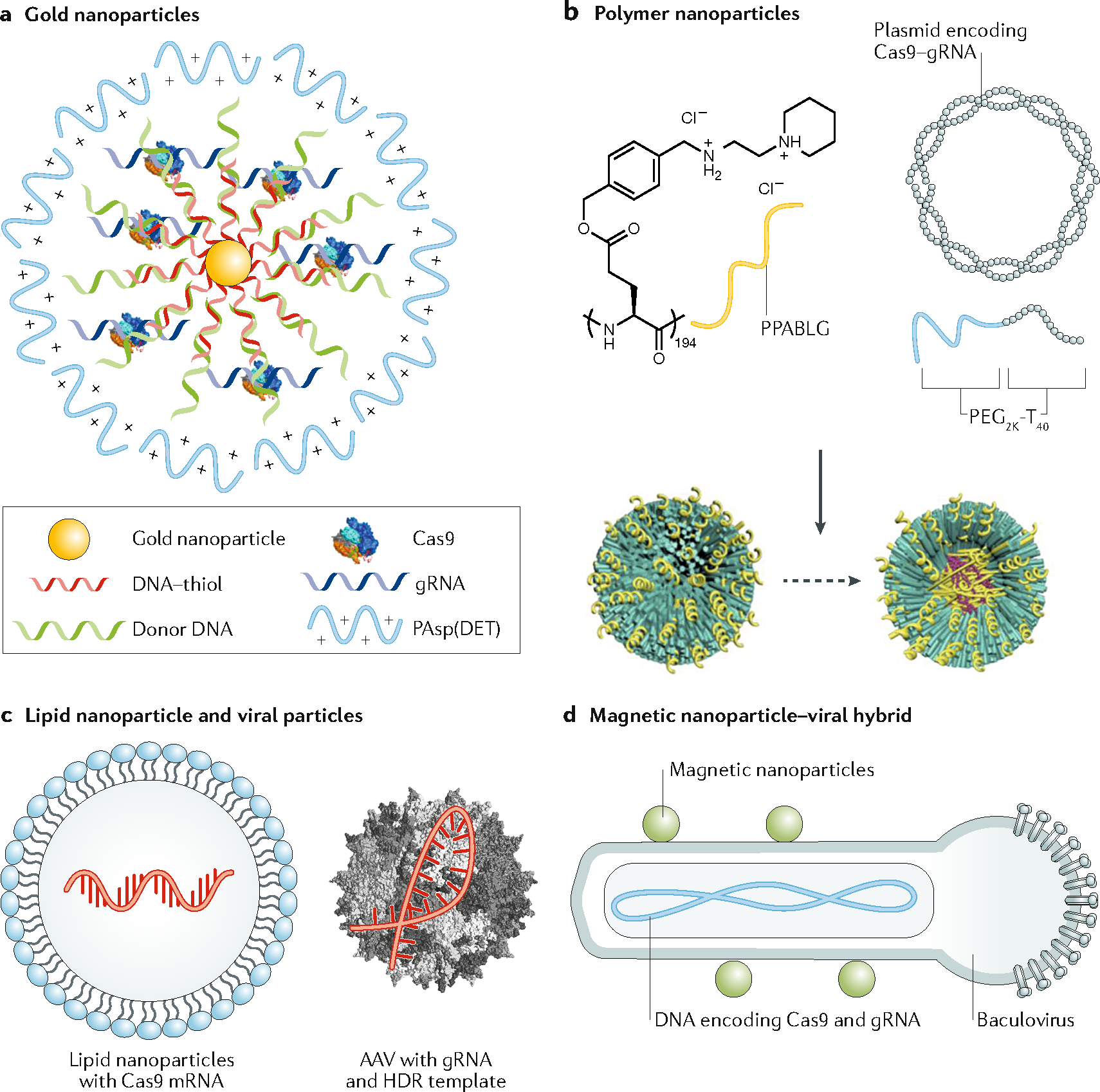
a. Delivery of Cas9/gRNA ribonucleoprotein (RNP) and the template for homology-directed repair (HDR) using gold nanoparticles (CRISPR–gold). CRISPR–gold is composed of gold nanoparticles conjugated with DNA, which are complexed with donor DNA, Cas9 RNP, and the polymer PAsp(DET) for endosomal disruption. Once in the cytoplasm, glutathione releases the DNA from the gold core of CRISPR–gold, which causes the rapid release of Cas9 RNP and donor DNA. b. Nanoparticle-based delivery of Cas9 plasmid and single guide RNA (gRNA). The positively charged α-helical polypeptide PPABLG complexes with Cas9 expression plasmids and gRNAs to form nanoparticles, which are then PEGylated (P-HNPs). P-HNPs can achieve efficient cellular internalization and endosomal escape. c. In vivo delivery of genome-editing machinery by combining lipid nanoparticles carrying Cas9 mRNA with AAV viral particles encoding gRNA and HDR donor template. d. In vivo delivery of CRISPR/Cas9 with spatial control of gene editing. DNA encoding Cas9 and gRNA is packaged into a baculoviral (BV) particle, which is complexed with magnetic nanoparticles (MNP–BV). By applying a magnetic field locally after delivery of MNP–BV, the inactivation of BV by the complement system in the serum can be overcome, leading to spatially controlled genome editing in the target tissue.
