Abstract
Purpose :
Fenestrated atrial septal defects (F-ASDs) in the pediatric population pose a challenge for transcatheter device closure since multiple devices are not preferred in small hearts. Oversizing the Amplatzer Septal Occluder (ASO) to cover the surrounding fenestrations usually distorts the central waist as well as the disc profile and often defeats the purpose. This is a retrospective observational study with an aim to assess the feasibility and safety of cribriform ASO in closing F-ASDs in small children.
Methods:
Sixteen children with F-ASD who underwent device closure with cribriform ASO were included in the study. The fenestrated septal length (FSL) and the total septal length (TSL) were measured on transesophageal echocardiogram. A device size which was 1.5–2 times the FSL but smaller than the TSL was selected. The defect was closed with a device passed through a relatively centrally placed smaller fenestration.
Results:
The median age of the cohort was 5 years (2.5–10.5). Majority (14/16) required 25 or 30 mm cribriform ASO. Aneurysmal interatrial septum was seen in most of our patients (11/15). All the patients had successful device implantation. Complete closure of the defect was seen in 11 patients while 5 patients had insignificant residual shunt at a median follow-up of 40 months (1–60 months). There were no other complications.
Conclusions:
Cribriform ASO can be used safely and effectively in closing F-ASDs in children. Deployment of the device through a small central hole allows covering maximum fenestrations and gives more stability to the device. Residual shunts, although not infrequent, are insignificant.
Keywords: Cribriform device, multifenestrated atrial septal defect, nonself-centering atrial septal defect device, septal aneurysm
INTRODUCTION
Transcatheter closure of secundum atrial septal defects (ASDs) is a well-accepted alternative to surgical repair in selected patients.[1,2] However, percutaneous closure of multifenestrated secundum ASD (F-ASD), which accounts for 10% of all ASDs,[3] is often challenging, especially in the pediatric age group.
In children with multiple ASDs, there are reports of successful placement of multiple devices[4,5,6] or deployment of a single device after performing a balloon atrial septostomy.[7] However, deployment of multiple devices increases the procedure time and more importantly the cost of the procedure, with a potential for embolization and erosion in a relatively small-sized heart.[5] Oversizing the Amplatzer Septal Occluder (ASO) to cover the surrounding holes usually distorts the central waist as well as disc profile, often leaving significant residual shunts, which defeats the purpose. The AMPLATZER™ Cribriform Multifenestrated Septal Occluder (Cribriform ASO) (Abbott Structural, Santa Clara, CA) is developed to address this very problem. It is a nonself-centering device with a small connecting waist diameter and large, equal-sized left and right atrial discs to cover more than one adjacent defect.[8]
There have been earlier reports of F-ASD closure using cribriform ASO in mixed (pediatric and adult) populations[9] but none exclusively in children. The aim of this study was to assess the feasibility and safety of this device in pediatric patients and to elaborate on technical considerations with regard to the selection of device size and deployment technique modifications which deviate slightly from the recommendations of the manufacturer.[8]
METHODS
This is a retrospective observational study involving children <18 years who underwent device closure using cribriform ASO between April 2014 and May 2019. The patient and procedural details were obtained from the hospital records. The F-ASD was defined as three or more defects (including fenestrations) in the interatrial septum (IAS) as seen on the transthoracic echocardiogram (TTE) or transesophageal echocardiogram (TEE) [Movie 1a and b].
All patients underwent physical examination, chest X-ray (PA), ECG, and TTE, and written informed consent was obtained from each patient's parent or guardian before transcatheter device closure. Although the patient selection was made on the basis of TTE, the final decision to use cribriform ASO including its size was made only after TEE evaluation during the procedure.
Inclusion criteria were as follows:
Patients with multiple fenestrations (≥3) with or without aneurysm of the IAS with the major defect <12 mm
Age <18 years
Signs of right ventricular overload
Presence of a rim of ≥4 mm from the defect to the superior or inferior vena cava, coronary sinus, mitral valve, and right upper pulmonary vein.
Exclusion criteria were as follows:
Coexisting cardiac lesions requiring surgical correction
One of the defects measuring >20 mm in diameter
Contraindication for antiplatelet therapy.
Implantation procedure
The procedure was done under general anesthesia. The two-dimensional (2D) TEE was performed for assessment of the defect as well as for guiding the device deployment.
The following measurements were obtained for assessment and device selection:
Fenestrated septal length (FSL): It was defined as the length of atrial septum which included all the openings and the aneurysm if present. In the presence of aneurysm, the length of the base of aneurysm was considered. This was measured in three standard imaging planes, namely, 0°, 45°, and 90° [Figure 1a–c]. The maximum measurement obtained in any of these views was labeled as FSL. The presence of aneurysm was noted using standard criteria[3,10]
Total septal length (TSL) [Figure 1a–c] : The maximum measurement of the IAS obtained in the above planes and views was considered as TSL.
Figure 1.
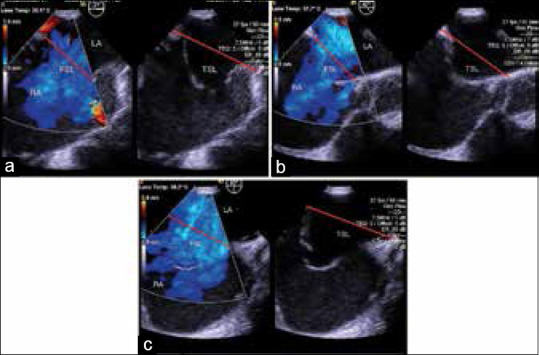
(a) Transesophageal echocardiographic image of four chamber view of fenestrated atrial septal defect with septal aneurysm demonstrating measured FSL and TSL. (b) Transesophageal echocardiographic image of short axis view of fenestrated atrial septal defect with septal aneurysm demonstrating measured FSL and TSL. (c) Transesophageal echocardiographic image of bicaval view of fenestrated atrial septal defect with septal aneurysm demonstrating measured fenestrated and total septal length. LA: Left atrium, RA: Right atrium, FSL: Fenestrated septal length, TSL: Total septal length
Device size selection was based on FSL and TSL. The device chosen was ≥1.5 times FSL but less than TSL. For example, if FSL was 12 mm and TSL was 28 mm, we selected an 18 or 25 mm device. In the presence of an aneurysm, there was a tendency to use larger device. However, if 150% of FSL was more than TSL, the device used was equal to or less than TSL.
After the initial TEE study, a single femoral venous access was obtained using the Seldinger technique. 100 IU/Kg of heparin was administered. The first dose of IV cefuroxime was given just before sending the patient to the laboratory. Oximetry run was done, and pulmonary arterial pressures were measured before proceeding with transcatheter ASD closure. The technique of device implantation was similar to that previously described[8] except that meticulous care was taken to cross the defect through the smaller but relatively central fenestration close to the major defect and not through the major defect itself (in contrast to the technique described in earlier studies and by the manufacturer).[8,9] TEE was used to guide crossing through the desired smaller central fenestration [Figure 2a and b] [Movie 2]. However, it was not always possible to ensure crossing the smaller fenestration, especially in the presence of aneurysmal IAS. In patients where we realized having inadvertently crossed a larger defect at the time of sheath or device deployment, a contralateral femoral venous access was taken and the smaller defect was recrossed keeping the first sheath assembly across the larger defect until the wire and catheter were across the smaller desired fenestration.
Figure 2.
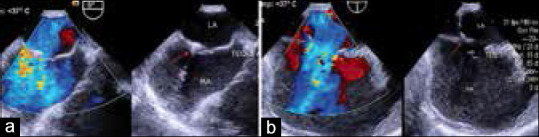
(a) Transesophageal echocardiographic images of four chamber view of the wire (Arrow) crossing the desired central, smaller defect. (b) Transesophageal echocardiographic images of bicaval view of the wire (Arrow) crossing the desired central, smaller defect. LA: Left atrium, RA: Right atrium
This deployment technique through the smaller hole allowed better device stability, this being a nonself centering device with a thin central waist. Using this technique, we were also able to deviate from the manufacturer's recommendation of requiring a minimum of 9 mm from the defect edge to the aortic root and superior vena cava (SVC).[8] By crossing the defect through a central fenestration, it was effectively ensured that the distance between the short central waist and SVC and aortic rims was adequate. It also helped in uniform and symmetrical disposition of the discs across the fenestrated portion of the IAS thereby covering most, if not all the fenestrations. Hence, with the device placed centrally, disc edges did not protrude into the SVC orifice or indent the aorta even in those of our patients who had additional peripheral fenestrations. The proximity of the central device waist to the major defect confirmed that it closed the major defect completely. The final device position was evaluated by TEE and once found to be suitable, the device was released. The position of the device in relation to the septum, its stability, and presence of residual shunts were evaluated by TEE after release [Figure 3a–c and Movie 3a and b].
Figure 3.
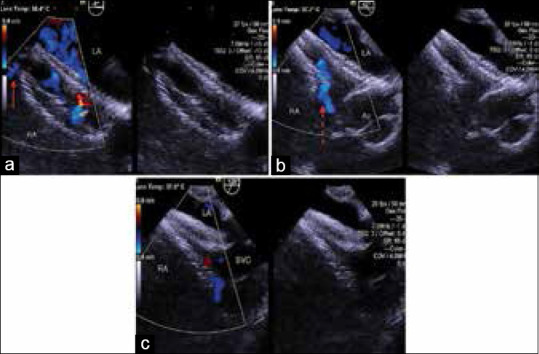
(a) Transesophageal echocardiographic images of the same child in four chamber view following closure of a fenestrated atrial septal defect with FSL of 26 mm and TSL of 32 mm using a 30 mm cribriform Amplatzer septal occluder. Note hemodynamically insignificant residual shunt (Arrow). (b) Transesophageal echocardiographic images of the same child in short axis view following closure of a fenestrated atrial septal defect with FSL of 26 mm and TSL of 32 mm using a 30 mm cribriform Amplatzer septal occluder. Note the foaming through the device (Dotted arrow). (c) Transesophageal echocardiographic images of the same child in bicaval view following closure of a fenestrated atrial septal defect with FSL of 26 mm and TSL of 32 mm using a 30 mm cribriform Amplatzer septal occluder. LA: Left atrium, RA: Right atrium, Ao: Aorta, SVC: Superior vena cava, FSL: Fenestrated septal length, TSL: Total septal length
Two additional doses of antibiotics were given at 8-h intervals. The ECG and TTE were performed on the following day before discharge from the hospital. Patients received low-dose (5 mg/kg) aspirin for 6 months. On the postdischarge, the child was evaluated clinically with an ECG and TTE with color Doppler at 6 weeks, 6 months, 12 months, and every year thereafter.
RESULTS
Between April 2014 and May 2019, 16 children (12 girls) with F-ASD underwent device closure using cribriform ASO. The demographic variables and defect characteristics are shown in Table 1. The median age was 5 years (range: 2.5–10.5 years). All the patients had evidence of hemodynamically significant left to right shunt (mean pulmonary to systemic flow ratio was 1.8:1). Eleven of 16 patients (69%) had an associated aneurysm of the IAS [Figure 1]. The mean FSL was 17.4 ± 5.5 mm while the TSL was 33.1 ± 3 mm. The mean ratio of FSL: TSL was 0.53:1. Seven of the 16 patients had an FSL/TSL ratio of <0.5, i.e., the fenestrated septum occupied a relatively small part of the atrial septum.
Table 1.
Demographic and procedural data
| Patient number | Age (years) | Gender | Aneurysm of IAS | Qp/Qs | FSL (mm) | TSL (mm) | Device size | Residual shunt |
|---|---|---|---|---|---|---|---|---|
| 1 | 3 | Female | Yes | 2.1 | 26 | 32 | 30 | No |
| 2 | 7 | Female | Yes | 1.8 | 17 | 38 | 30 | No |
| 3 | 5 | Female | No | 1.9 | 14 | 30 | 25 | No |
| 4 | 2.5 | Male | Yes | 2 | 19 | 36 | 30 | Yes |
| 5 | 9 | Female | Yes | 1.4 | 12 | 32 | 18 | No |
| 6 | 3 | Female | Yes | 1.4 | 16 | 31 | 25 | No |
| 7 | 6 | Male | No | 1.9 | 17 | 32 | 25 | Yes |
| 8 | 5 | Female | Yes | 2.1 | 14 | 30 | 25 | No |
| 9 | 9 | Male | Yes | 1.7 | 19 | 36 | 30 | No |
| 10 | 5 | Female | Yes | 1.7 | 16 | 30 | 25 | No |
| 11 | 4 | Female | Yes | 2 | 22 | 28 | 25 | Yes |
| 12 | 6 | Female | No | 1.9 | 16 | 33 | 25 | No |
| 13 | 8 | Male | No | 1.6 | 15 | 35 | 25 | No |
| 14 | 3 | Female | Yes | 1.8 | 22 | 30 | 25 | No |
| 15 | 5 | Female | Yes | 2 | 17 | 40 | 35 | Yes |
| 16 | 10.5 | Female | No | 1.7 | 15 | 38 | 25 | Yes |
Patient number (16) had 2 devices - one 25 mm cribriform ASO and 16 mm ASO. IAS: Interatrial septum, FSL: Fenestrated septal length, TSL: Total septal length, ASO: Amplatzer septal occluder
The sizes of the device used were 18 mm in one patient, 25 mm in 10 patients, 30 mm in 4 patients, and 35 mm in one patient, respectively. One patient had a large defect in the region of fossa ovalis and multiple tiny fenestrations in the posteroinferior part of the IAS with significant distance between the two. The patient required two devices as follows: the large defect was closed using a 16 mm ASO while the fenestrated portion was closed using a 25 mm cribriform ASO. Three patients needed recrossing of the defect from the contralateral femoral access as the initial crossing was not through the desirable central small hole. Of these three, two needed recrossing after placement of the sheath and one after deployment of the device. Complete closure was achieved immediately in 9 (56.2%) which increased to 11 patients (68%) at the time of the last follow-up. At most recent follow-up, five patients continue to show small, insignificant residual shunt of <3 mm in diameter on transthoracic color Doppler echo through one of the fenestrations. All these patients had normal right heart dimensions on echocardiogram and absence of cardiomegaly on chest X-ray. The follow-up period ranged from 1 to 60 months (median: 40 months). There were no early or late complications such as malposition/embolization, arrhythmias, thromboembolism, atrioventricular valve dysfunction, systemic or pulmonary venous obstruction, erosion, pericardial effusion, cardiac tamponade, or death.
DISCUSSION
This study is the first one to evaluate the feasibility and safety of F-ASD device closure using a cribriform ASO in pediatric patients. All our patients had successful deployment of the cribriform ASO with no major complications in the short and intermediate term.
At present, apart from the use of cribriform ASO, three other strategies are employed for transcatheter closure of F-ASDs. The first one involves the simultaneous implantation of two or more ASOs.[4,5,11] This usually requires the distance between two adjacent holes to be ≥7 mm, and it is recommended that the smaller device should be delivered first, with the larger device overlapping the smaller one.[11,12] The use of multiple devices in children with relatively smaller hearts may predispose to device embolization and cardiac erosion.[5] Furthermore, there is no experimental or clinical data on endothelialization of the Amplatzer prosthesis when two devices overlap each other, but the absence of endothelialization is plausible if part of a device disc has no contact with the endocardial surface of the atrial septum.[13] This may predispose to clot formation after cessation of antiplatelet drugs in such patients or even infective endocarditis.[14]
The second strategy consists of a balloon atrial septostomy to tear the strands separating the two or more defects thereby creating a confluence between the holes and thereafter placing a single larger ASO.[7] The balloon atrial septostomy is a hazardous maneuver because the new larger hole is not regularly elliptical in shape, and the resulting estimated balloon sizing is unpredictable, leading to undersizing or oversizing of the generated defect.[7]
Both these strategies increase the procedure time and the cost. The third strategy is to close the F-ASD with a single ASO if the distance of the smaller defects from the main defect is <7 mm.[15] In many such cases, all the defects get closed with a single oversized device or in some, a small residual defect with hemodynamically insignificant shunt is left behind.[15,16] However, this strategy is unlikely to work if there are multiple satellite defects which are farther than 7 mm from the main defect resulting in a significant residual shunting. Some authors have also performed a central puncture in the IAS to successfully close multiple ASDs using the standard ASO.[17] However, atrial septal puncture if there is redundant aneurysmal IAS can be technically challenging and potentially risky. Furthermore, serious complications such as atrioventricular valve damage, erosion resulting in cardiac tamponade, and death have been reported with larger devices if one adopts a single oversized device strategy for closing large F-ASD.[18,19,20,21]
Selecting the device size for cribriform ASO
In F-ASD with the normal septum, the device size was determined by the FSL and the TSL. We used a device which was ≥1.5 times larger than the length of the fenestrated septum but less than or equal to the length of the IAS. Numan et al. selected a device that was at least 5.5 mm larger than the steady rim or a device to steady rim ratio of 1.4:1.[9] The manufacturer recommends that after crossing the central fenestration, the shortest distance from the central defect to the aortic root or the SVC should be measured and the device should be selected as shown in their sizing chart.[8]
In F-ASD with aneurysmal IAS, as seen in 11 of our 16 patients, we used a little larger device if the TSL permitted. This helped in squeezing almost the entire aneurysmal tissue between the discs thereby closing all the fenestrations, especially the ones at the end of the aneurysm. Furthermore, the bunched-up aneurysmal tissue offered extra stability to the device.
Principles of device deployment
In F-ASD, which fenestration should serve as the anchoring point of the central waist of cribriform ASD is a matter of debate. In the present study, we preferred to cross the defect and deploy the device through one of the smaller central defects of the fenestrated portion just adjacent to the major defect instead of the major defect itself. In contrast to our approach, most of the previously reported studies recommend crossing the central fenestration while using the cribriform ASO,[8,9,13] among which some specifically mention about crossing the central major fenestration.[8,9] We believe that our strategy offers greater stability to the device and would reduce the chances of migration in the absence of a large self-centering waist. This hypothesis is supported by the fact that all the patients had stable device position without any embolization or displacement. Furthermore, being in close proximity of the major defect, it covered the major defect adequately in all the patients. Crossing the defect through the center of the fenestrated portion helped in covering the maximum number of defects thereby reducing the incidence and quantum of the residual shunt.
Although we tried to cross the smaller of the defects in the center, it may not always be possible to define where the catheter has crossed on 2D TEE till such time as the sheath crosses through or on some occasions till the device is deployed. Given the age and weight of these patients, 3D TEE could not be used in this subset of patients due to the weight limitations of the 3D TEE probe. Under these circumstances, one may need to recross the defect to achieve the desired site of deployment. In this study, we had to recross the defect in three patients through the contralateral femoral vein keeping in place the sheath which had crossed through the wrong defect. Zanchetta et al. used a double-guidewire technique whereby they placed two guidewires across the fenestrated septum and then used intracardiac echo (ICE) to choose the one which was central in position.[13] Unfortunately, we had no access to ICE.
We used two devices in only one patient because of a long distance between the major defect and the fenestrated portion of the IAS. Cho et al. used cribriform device in combination with ASO in their patient with multiple ASD.[22] They used the cribriform device because the wire had crossed the smaller of the 3 ASDs. Another ASO was deployed for the remaining ASD.[22]
CONCLUSIONS
F-ASDs can be effectively and safely closed in the pediatric population using the cribriform ASO. Our suggested strategy of deployment of the cribriform ASO through a small central hole, although slightly different from the manufacturer's recommendation, allows covering maximum fenestrations and gives more stability to the device. Residual shunts, although not infrequent, are insignificant with this technique.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Movies Available on: www.annalspc.com
REFERENCES
- 1.Berger F, Ewert P, Björnstad PG, Dähnert I, Krings G, Brilla-Austenat I, et al. Transcatheter closure as standard treatment for most interatrial defects: Experience in 200 patients treated with the Amplatzer Septal Occluder. Cardiol Young. 1999;9:468–73. doi: 10.1017/s1047951100005369. [DOI] [PubMed] [Google Scholar]
- 2.Butera G, De Rosa G, Chessa M, Rosti L, Negura DG, Luciane P, et al. Transcatheter closure of atrial septal defect in young children: Results and follow-up. J Am Coll Cardiol. 2003;42:241–5. doi: 10.1016/s0735-1097(03)00589-8. [DOI] [PubMed] [Google Scholar]
- 3.Podnar T, Martanovic P, Gavora P, Masura J. Morphological variations of secundum-type atrial septal defects: Feasibility for percutaneous closure using Amplatzer septal occluders. Catheter Cardiovasc Interv. 2001;53:386–91. doi: 10.1002/ccd.1187. [DOI] [PubMed] [Google Scholar]
- 4.Yang Y, Xu Z, Jiang S, Zhao S, Zhang G, Jin J, et al. Simultaneous transcatheter closure of multiple atrial septal defects using dual amplatzer septal occluder devices. Am J Med Sci. 2016;352:245–51. doi: 10.1016/j.amjms.2016.05.029. [DOI] [PubMed] [Google Scholar]
- 5.Awad SM, Garay FF, Cao QL, Hijazi ZM. Multiple Amplatzer septal occluder devices for multiple atrial communications: Immediate and long-term follow-up results. Catheter Cardiovasc Interv. 2007;70:265–73. doi: 10.1002/ccd.21145. [DOI] [PubMed] [Google Scholar]
- 6.Mahadevan VS, Gomperts N, Haberer K, Silversides C, Benson LN, McLaughlin PR, et al. Transcatheter closure of atrial septal defects with multiple devices in adults: Procedural and clinical outcomes. Int J Cardiol. 2009;133:359–63. doi: 10.1016/j.ijcard.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 7.Carano N, Hagler DJ, Agnetti A, Squarcia U. Device closure of fenestrated atrial septal defects: Use of a single Amplatz atrial septal occluder after balloon atrial septostomy to create a single defect. Catheter Cardiovasc Interv. 2001;52:203–7. doi: 10.1002/1522-726x(200102)52:2<203::aid-ccd1048>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 8.St Jude Medical (Abbott structural) Product Manual: AMPLATZER Multifenestrated Septal Occluder- “Cribriform”. Instructions for Use. 2020. [Last acessed on 2020 Jul 13]. Available from: https://manuals.sjm.com/~/media/manuals/product-manual-pdfs/1/9/19317d63-3146-4382-927f-a27e2d85ea89.pdf .
- 9.Numan M, El Sisi A, Tofeig M, Gendi S, Tohami T, El-Said HG. Cribriform amplatzer device closure of fenestrated atrial septal defects: Feasibility and technical aspects. Pediatr Cardiol. 2008;29:530–5. doi: 10.1007/s00246-007-9079-x. [DOI] [PubMed] [Google Scholar]
- 10.Hanley PC, Tajik AJ, Hynes JK, Edwards WD, Reeder GS, Hagler DJ, et al. Diagnosis and classification of atrial septal aneurysm by two-dimensional echocardiography: Report of 80 consecutive cases. J Am Coll Cardiol. 1985;6:1370–82. doi: 10.1016/s0735-1097(85)80228-x. [DOI] [PubMed] [Google Scholar]
- 11.Pedra CA, Fontes-Pedra SR, Esteves CA, Assef J, Fontes VF, Hijazi ZM. Multiple atrial septal defects and patent ductus arteriosus: Successful outcome using two Amplatzer septal occluders and Gianturco coils. Cathet Cardiovasc Diagn. 1998;45:257–9. doi: 10.1002/(sici)1097-0304(199811)45:3<257::aid-ccd8>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 12.Cao Q, Radtke W, Berger F, Zhu W, Hijazi ZM. Transcatheter closure of multiple atrial septal defects. Initial results and value of two- and three-dimensional transoesophageal echocardiography. Eur Heart J. 2000;21:941–7. doi: 10.1053/euhj.1999.1909. [DOI] [PubMed] [Google Scholar]
- 13.Zanchetta M, Rigatelli G, Pedon L, Zennaro M, Carrozza A, Onorato E. Catheter closure of perforated secundum atrial septal defect under intracardiac echocardiographic guidance using a single amplatzer device: Feasibility of a new method. J Invasive Cardiol. 2005;17:262–5. [PubMed] [Google Scholar]
- 14.Slesnick TC, Nugent AW, Fraser CD, Jr, Cannon BC. Images in cardiovascular medicine. Incomplete endothelialization and late development of acute bacterial endocarditis after implantation of an Amplatzer septal occluder device. Circulation. 2008;117:e326–7. doi: 10.1161/CIRCULATIONAHA.107.754069. [DOI] [PubMed] [Google Scholar]
- 15.Szkutnik M, Masura J, Bialkowski J, Gavora P, Banaszak P, Kusa J, et al. Transcatheter closure of double atrial septal defects with a single Amplatzer device. Catheter Cardiovasc Interv. 2004;61:237–41. doi: 10.1002/ccd.10753. [DOI] [PubMed] [Google Scholar]
- 16.Tal R, Dahud Q, Lorber A. Fenestrated atrial septal defect percutaneously occluded by a single device: Procedural and financial considerations. Cardiol Ther. 2013;2:97–102. doi: 10.1007/s40119-012-0009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jihun A, Hoi KD. TCTAP C-241 Transcatheter closure of atrial septal aneurysm associated with multiple atrial septal defect. J Am Coll Cardiol. 2016;67:5395–96. [Google Scholar]
- 18.Perry YY, Triedman JK, Gauvreau K, Lock JE, Jenkins KJ. Sudden death in patients after transcatheter device implantation for congenital heart disease. Am J Cardiol. 2000;85:992–5. doi: 10.1016/s0002-9149(99)00916-9. [DOI] [PubMed] [Google Scholar]
- 19.Berger F, Vogel M, Alexi-Meskishvili V, Lange PE. Comparison of results and complications of surgical and Amplatzer device closure of atrial septal defects. J Thorac Cardiovasc Surg. 1999;118:674–8. doi: 10.1016/S0022-5223(99)70013-9. [DOI] [PubMed] [Google Scholar]
- 20.Chessa M, Carminati M, Butera G, Bini RM, Drago M, Rosti L, et al. Early and late complications associated with transcatheter occlusion of secundum atrial septal defect. J Am Coll Cardiol. 2002;39:1061–5. doi: 10.1016/s0735-1097(02)01711-4. [DOI] [PubMed] [Google Scholar]
- 21.Du ZD, Hijazi ZM, Kleinman CS, Silverman NH, Larntz K Amplatzer Investigators. Comparison between transcatheter and surgical closure of secundum atrial septal defect in children and adults: Results of a multicenter nonrandomized trial. J Am Coll Cardiol. 2002;39:1836–44. doi: 10.1016/s0735-1097(02)01862-4. [DOI] [PubMed] [Google Scholar]
- 22.Cho MJ, Song J, Kim SJ, Choi EY, Lee SY, Shim WS. Transcatheter closure of multiple atrial septal defects with the amplatzer device. Korean Circ J. 2011;41:549–51. doi: 10.4070/kcj.2011.41.9.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


