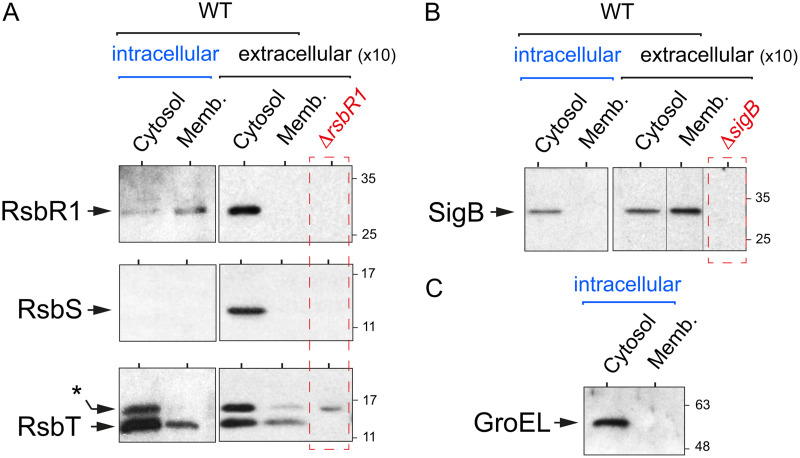
FIG 2.
The core stressosome protein RsbR1 is predominantly associated with the membrane in intracellular L. monocytogenes. JEG-3 epithelial cells were infected for 30 min with L. monocytogenes EGD-e wild type (WT) at a multiplicity of infection of 10:1 (bacteria to epithelial cells). Noninternalized bacteria were removed by washing steps, and 10 μg/ml gentamicin was added to the medium. Infected cells were incubated up to 6 h postinfection. At this time, intracellular bacteria were recovered in epithelial cell lysates by high-speed centrifugation. Cytosolic and membrane extracts were prepared from intracellular bacteria (6 hpi) and from extracellular bacteria grown in BHI medium (optical density at 600 nm [OD600] ≈ 0.4), as described elsewhere (28). The extracellular samples corresponded to 10-fold more bacteria. (A) Levels of the stressosome core proteins RsbR1 and RsbS and the kinase RsbT in intra- and extracellular bacteria. *, unspecific band detected in the null ΔrsbR1 polar mutant, as described in reference 28. (B) Levels of the sigma factor SigB. (C) Control assay for the relative enrichment of the cytosolic/membrane fractions based on the detection of the cytosolic chaperonin GroEL. Shown are immunoblots using the respective antibodies raised against each of the indicated proteins. Data correspond to a representative experiment from a minimum of three biological replicates.