ABSTRACT
Tyrosinase is a bifunctional enzyme mediating the o-hydroxylation and two-electron oxidation of monophenols to o-quinones. The monophenolase activity of tyrosinase is much desired for the industrial synthesis of catechols. However, the generally low ratio of monophenolase/diphenolase activity of tyrosinase limited its utilization in the industry. In this study, a novel tyrosinase from Armillaria ostoyae strain C18/9 (AoTyr) was characterized, and the results showed that the enzyme has an optimal temperature of 25°C and an optimal pH of 6. The enzyme has comparable monophenolase and diphenolase activities and exhibits substrate inhibition in both of the activities. In silico analysis and mutagenesis experiments showed that residues 262 and 266 play important roles in modulating the substrate inhibition and enzymatic activities of AoTyr, and the replacement of D262 with asparagine significantly increased the monophenolase/diphenolase catalytic efficiencies (kcat/Km ratios) (1.63-fold) of the enzyme. The results from this study indicated that this novel tyrosinase could be a potential candidate for the industrial biosynthesis of catechols.
IMPORTANCE Tyrosinase is able to oxidize various phenolic compounds, and its ability to convert monophenols into diphenols has caught great attention in the research field and industrial applications. However, the utilization of tyrosinase for the industrial synthesis of catechols has been limited due to the fact that the monophenolase activity of most of the known tyrosinases is much lower than the diphenolase activity. In the present study, a novel tyrosinase with comparable monophenolase and diphenolase activities was characterized. The enzyme exhibits substrate inhibition in both monophenolase and diphenolase activities. In silico analysis followed by mutagenesis experiments confirmed the important roles of residues 262 and 266 in the substrate inhibition and activity modulation of the enzyme, and the D262N variant showed an enhanced monophenolase/diphenolase catalytic efficiency ratio compared to the wild-type enzyme.
KEYWORDS: tyrosinase, Armillaria ostoyae, substrate inhibition, mutagenesis, diphenolase, monophenolase
INTRODUCTION
Tyrosinase (EC 1.14.18.1) is a type 3 copper enzyme containing a binuclear copper center and is also known as a polyphenol oxidase. In the presence of oxygen, tyrosinase drives two successive catalytic processes: monophenol hydroxylation and the subsequent o-diphenol oxidation (1). Its oxidation activity does not require any reducing cofactors such as NAD(P)H, unlike most oxygenases, including flavin-dependent monooxygenase and cytochrome P450 oxidase (2, 3). Tyrosinases have a wide distribution in almost all organisms and maintain essential physiological functions (4–8). The enzyme was first characterized in mammals, where it is involved in the development of melanomas and pigmentation disorders such as albinism and vitiligo (9). In sponges, plants, and many invertebrates, it is an important component of wound healing and the primary immune response (4, 10, 11). In arthropod processes such as sclerotization, it plays the role of the cuticle after molting or injury (12, 13). In fungi, it is mainly associated with browning and pigmentation (14).
The catalytic activities of tyrosinase may be applicable to various industrial efforts (15–17), and its ability to convert monophenols into diphenols has caught great attention in the research field since various produced o-diphenols are important precursors for the synthesis of agrochemicals, flavors, pharmaceuticals, polymerization inhibitors, antioxidants, and inks (18–22). Enzymatic methods have great advantages in the synthesis of catechols compared to traditional chemical methods, which require aggressive reagents and several reaction conditions and have poor yields (23). However, the utilization of tyrosinase for the industrial synthesis of catechols has been limited due to the fact that the monophenolase activity of most of the known tyrosinases is much lower than the diphenolase activity (24), and the accumulation of catechols during its two successive catalytic processes is not favored (25). Moreover, catechols such as 3,4-dihydroxyphenylalanine (DOPA) are unstable at relatively high pHs and high temperatures (26). Therefore, the search for novel tyrosinases with a highly enhanced monophenolase/diphenolase activity ratio and high activity under low-temperature and acidic pH conditions could be of great desire (27, 28).
In the present study, a novel tyrosinase from the fungus Armillaria ostoyae strain C18/9 (AoTyr) has been cloned and heterologously expressed in an Escherichia coli system. Amino acid sequence analysis indicated that it is a typical fungal tyrosinase containing a C-terminal extensional domain and a type 3 copper active center. The enzyme prefers acidic reaction conditions and relatively low temperatures. Activity assays showed that the monophenolase and diphenolase activities of the enzyme are comparable, indicating its potential use in the industrial synthesis of catechols. Moreover, it exhibits substrate inhibition in both of the activities. In silico analysis showed that residues D262 and D266 are involved in substrate recognition and binding, and their important role in the substrate inhibition of the enzyme has been further confirmed by mutagenesis. The effects of metal ions, detergents, reducing reagents, and inhibitors on enzyme activity were also evaluated.
RESULTS
Cloning and in silico analysis.
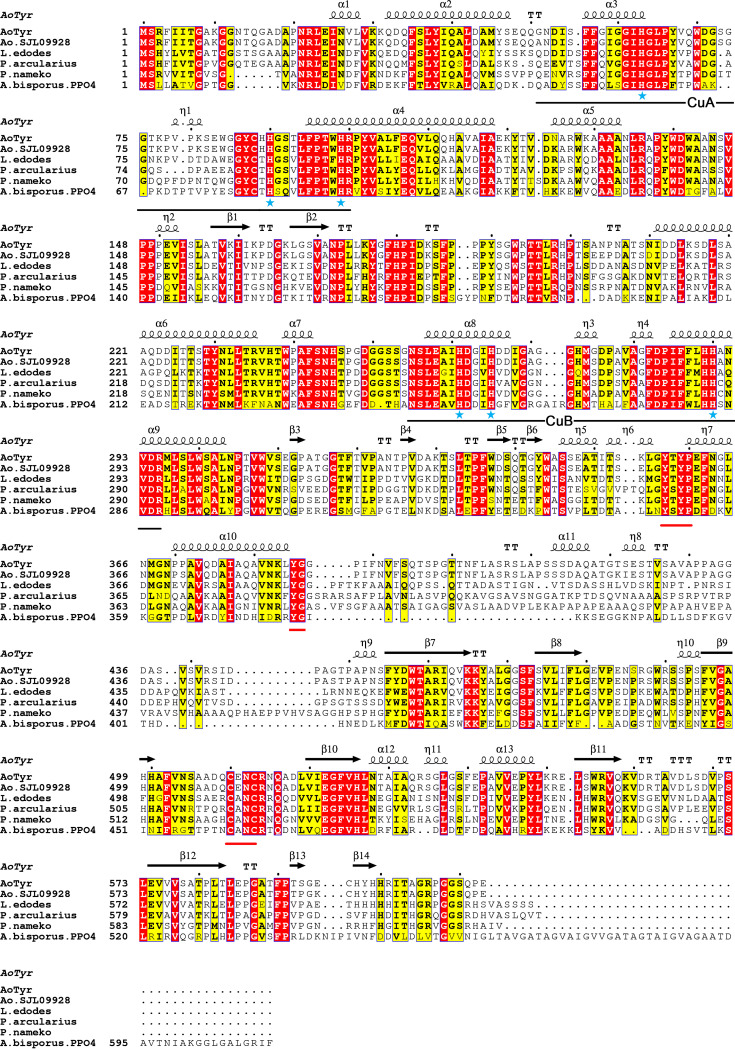
A novel tyrosinase gene was subcloned from A. ostoyae and named AoTyr. Multiple-sequence alignment showed the high sequence similarity of AoTyr with several polyphenol oxidases (PPOs), such as PPO4 from Agaricus bisporus (AbPPO4) (UniProt accession number C7FF05) (43.7% similarity) (29, 30), tyrosinase from Lentinus edodes (UniProt accession number Q96TI3) (66.6% similarity), tyrosinase from Pholiota nameko (UniProt accession number A7BHQ9) (62.6% similarity) (31), and photoregulated tyrosinase from Polyporus arcularius (UniProt accession number Q65Z70) (65.2% similarity) (Fig. 1). It is clear that the sequence of AoTyr can be divided into two different domains, namely, the N-terminal domain and the C-terminal domain, which are separated at the end of the α10 helix (Fig. 1). The N-terminal domain of AoTyr is mainly composed of the α-helix, which is characterized by the presence of several highly conserved motifs. A typical type 3 copper-binding motif (32) can be observed in the N-terminal sequence of AoTyr, in which CuA is coordinated by residues H63, H90, and H99 and CuB is coordinated by H261, H265, and H290 (marked by blue stars in Fig. 1). Downstream of the CuB site, a tyrosine motif (Y357-X-Y359) (8) and a following YG motif (33) also exist (segments marked with red underlining in Fig. 1). The C-terminal domain varies between tyrosinases, especially tyrosinases of different origins (Fig. 1) (34). However, the well-conserved CXXC motif (C510 to C513) is observed in this region in AoTyr (segments marked with red underlining in Fig. 1), which is essential for the incorporation of copper ions into the active site and the three-dimensional (3D) structure of the enzyme due to appropriate disulfide bonds (35, 36).
FIG 1.
Multiple-sequence alignments between AoTyr and highly homologous tyrosinases from A. ostoyae (GenBank accession number SJL09928.1), L. edodes (UniProt accession number Q96TI3), P. arcularius (UniProt accession number Q65Z70), P. nameko (UniProt accession number A7BHQ9), and A. bisporus (UniProt accession number C7FF05). Histidine residues participating in copper coordination are marked by blue stars, and conserved regions such as the type 3 copper-binding motif, the Y-X-Y motif, the YG motif, and the C-X-X-C motif are underlined.
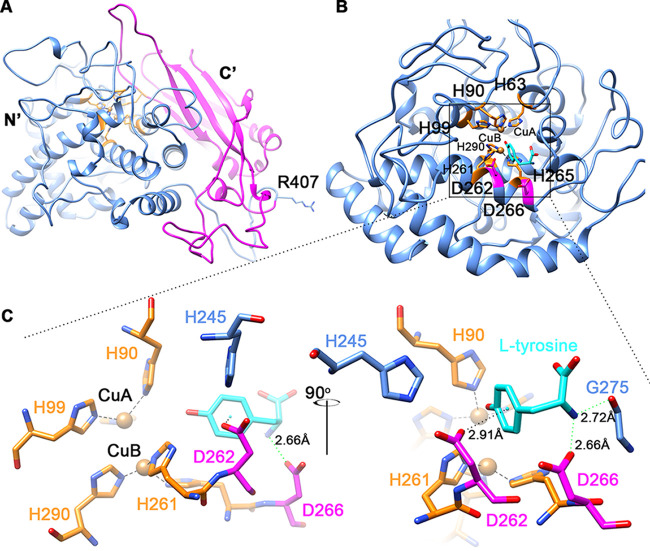
Based on the amino acid sequence, a 3D structure of AoTyr was built with the SWISS-MODEL online sever (37) using the crystal structure of AbPPO4 (PDB accession number 5M6B) (30) as the template (Fig. 2A). The obtained model has a global model quality estimation (a number between 0 and 1, with a higher number indicating higher reliability) (37) of 0.75 and a QMEAN (Qualitative Model Energy ANalysis) Z-score (38) (a score of −4.0 or below is an indication of a model with low quality) of −3.53, indicating the reliability of the model. As shown in the model, the N-terminal catalytic domain (Fig. 2A, blue) and the C-terminal extension domain (Fig. 2A, magenta) can easily be identified.
FIG 2.
Structure analysis of AoTyr. (A) Modeled latent AoTyr structure. The N-terminal (N′) domain is in blue, the C-terminal (C′) domain is marked in magenta, and the histidine residues involved in type 3 copper binding are depicted in orange. (B and C) View of the active site of the N-terminal domain. An l-tyrosine molecule (in cyan) is docked in the active center. Residues involved in copper coordination are colored and labeled in orange, and residues D262 and D266 are labeled and colored in magenta.
A docking study with l-tyrosine as the ligand showed that the ligand binds in a position similar to that observed in the Bacillus megaterium tyrosinase (TyrBm) (32). The side chain of residue D266 establishes a hydrogen bond with the amino group of l-tyrosine, which also forms a hydrogen bond with the carbonyl oxygen of residue G275, whereas the side chain of residue D262 formed a π-anion interaction with the phenol group of l-tyrosine (Fig. 2C). This suggests that residues D262 and D266 may play important roles in ligand recognition and binding. Moreover, the literature shows that N205 (equivalent to residue D262 in AoTyr) in TyrBm participated in water molecule activation during the reaction (32), and R209 (equivalent to residue D266 in AoTyr) in TyrBm plays an important role in the modulation of the monophenolase and diphenolase activities of the enzyme (39). Therefore, in the structure of AoTyr, the roles of D262 and D266 in substrate binding and activity modulation need to be evaluated.
Coexpression, purification, and activation of AoTyr.
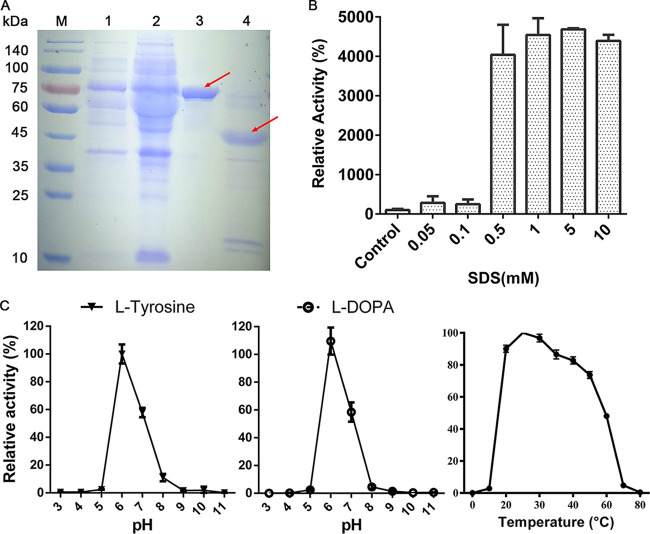
To prevent the formation of an inclusion body, the expression temperature was adjusted to 20°C, and the ratio of isopropyl-β-d-1-thiogalactopyranoside (IPTG) to l-arabinose was maintained at 1:500. The soluble latent AoTyr in cell lysis was purified by a Ni-Sepharose 6 FF column, resulting in a purified fraction with a prominent band at ∼66 kDa on a sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel (Fig. 3A). The activation of latent AoTyr was performed with both proteolysis and SDS treatment. The digestion of latent AoTyr with trypsin led to a reduction of the molecular weight to ∼46 kDa (Fig. 3B). Together with the results of ExPASy Peptide Cutter (40) calculation, the trypsin cleavage site was predicted to be located at R407. The activation of latent AoTyr by various concentrations of SDS showed that the activity of AoTyr increased dramatically when the SDS concentration reached 0.5 mM (Fig. 3B), and the highest activity was recorded at 5 mM SDS. A further increase of the SDS concentration exhibited an inhibitory effect on the activity.
FIG 3.
(A) SDS-PAGE analysis of AoTyr after Ni affinity chromatography. Lanes: M, molecular weight marker; 1, cell before induction; 2, cell after induction; 3, latent AoTyr purified by Ni affinity chromatography (eluted with 200 mM imidazole in buffer); 4, activated AoTyr (activation with trypsin). The weights of the used standard protein are indicated at the left of the gel. Protyrosinase (66 kDa) in lane 3 and tyrosinase (46 kDa) in lane 4 are indicated with red arrows. (B) SDS activation of latent AoTyr. For the control, the same amount of water was used as SDS. (C) pH and temperature profile of activated AoTyr. The substrate used in both pH and temperature profile assays was l-DOPA at a final concentration of 1 mM.
Effect of pH and temperature on AoTyr activity.
The enzyme has similar pH profiles of both monophenolase and diphenolase activities (Fig. 3C). The active pH ranges for both activities are narrow, with the same optimal pH of 6. The enzyme becomes almost inactive when the pH decreases to 5 or increases to 8, and it maintains about 60% of the activities at pH 7. As for the temperature profile, AoTyr had an optimal temperature of 25°C and maintained most of the enzymatic activity up to 60°C, indicating the high thermotolerance of the enzyme (Fig. 3C).
Effect of organic solvents on AoTyr activity.
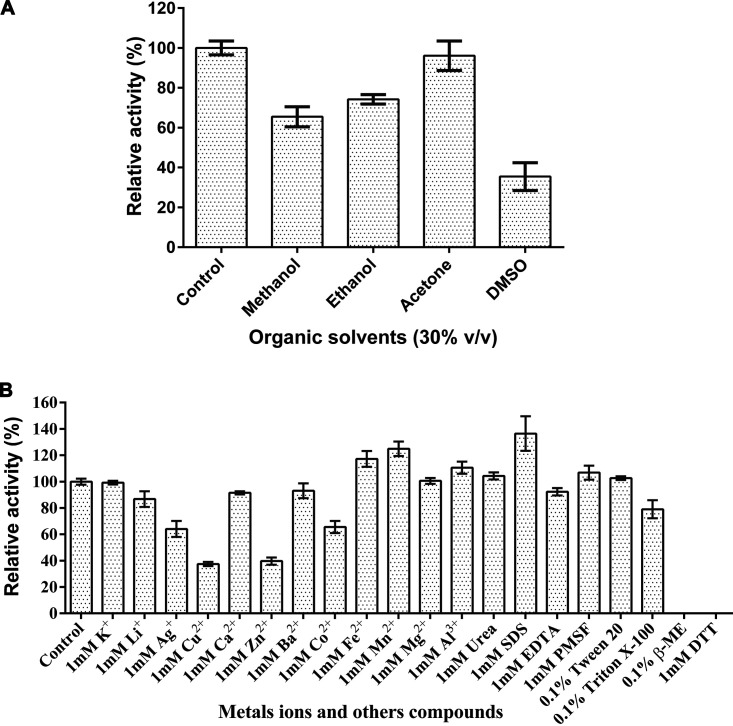
The presence of 30% (vol/vol) methanol (65.5% ± 5.04%), ethanol (74.2% ± 2.36%), or dimethyl sulfoxide (DMSO) (35.5% ± 7.00%) in the reaction mixture significantly inhibited the activity of AoTyr (Fig. 4A). Surprisingly, no significant effect was observed with 30% (vol/vol) acetone.
FIG 4.
Effects of organic solvents (A), metal ions, and other compounds (B) on AoTyr activity. In the reaction mixtures, 1 mM l-DOPA was used as the substrate. For the control, the same amount of water was used as the chemicals or agents.
Effect of metal ions, detergents, and inhibitors on AoTyr activity.
Direct inactivation of the enzyme was observed in the presence of reducing reagents such as β-mercaptoethanol (β-ME) and dithiothreitol (DTT), and a remarkable inhibitory effect was also observed in the presence of several metal ions such as Cu2+ (37.4% ± 2.78%) and Zn2+ (39.7% ± 4.71%) (Fig. 4B). In contrast, Fe2+ (117.3% ± 10.4%), Mn2+ (124.9% ± 9.61%), and Al3+ (110.6% ± 7.96%) showed a significant stimulatory effect on the activity of AoTyr. Ions such as Ag+ (64.1% ± 10.5%) and Co2+ (65.6% ± 7.94%) had a mild inhibitory effect on AoTyr activity. As for detergents, SDS (136.4% ± 22.7%) promoted the enzyme activity significantly, while Triton X-100 (79.0% ± 11.8%) showed an inhibitory effect instead. To our surprise, the presence of 1 mM EDTA (92.3% ± 4.78%) showed a limited effect on AoTyr activity.
Stereoselectivity and substrate specificity of AoTyr.
The relative activity of AoTyr toward d-tyrosine was only 39.3% ± 2.20% of its activity toward l-tyrosine. As for the substrate specificities of the enzyme, it acts on all tested monophenols, including phenol, 2,4-dimethylphenol, 3,4-dimethylphenol, and 2,3,5-trimethylphenol (Fig. 5). The benzoquinones were the predominant products when using phenol and 3,4-dimethylphenol as the substrates (Fig. 5A and C). When 2,4-dimethylphenol was used as the substrate, the produced 3,5-dimethyl-1,2-benzoquinone (retention time of 3.46 min) and 3,5-dimethylcatechol (retention time of 29.7 min) were observed in the product spectra, with area under curve (AUC) values of 608 and 676, respectively (Fig. 5B). Similarly, the produced 3,4,6-trimethylcyclohexa-3,5-diene-1,2-dione (retention time of 3.39 min) and 3,4,6-trimethylcatechol (retention time of 45.0 min) from 2,3,5-trimethylphenol can also be observed in the high-performance liquid chromatography (HPLC) spectra with AUC values of 582 and 262, respectively (Fig. 5D).
FIG 5.
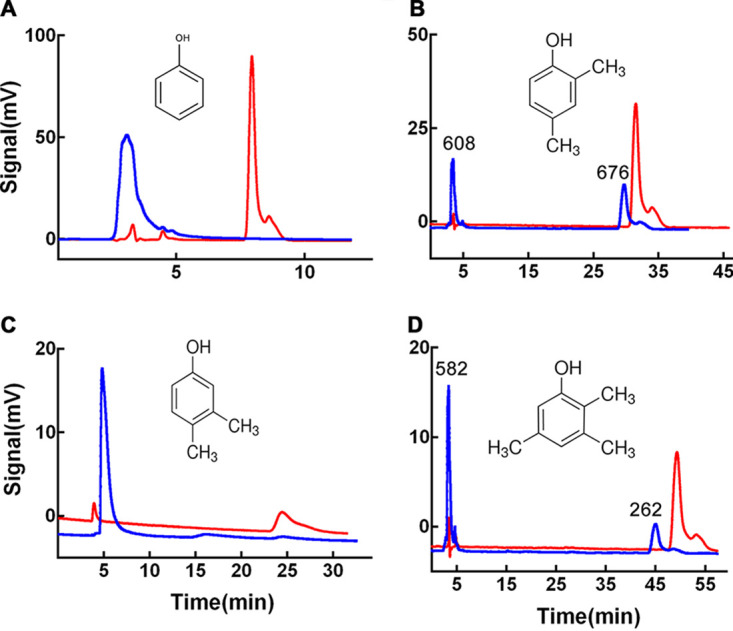
Substrate specificity of trypsin-activated AoTyr toward different monophenols. Product analysis was performed using HPLC at 270 nm. The red lines represent substrates before the catalytic reaction, and the blue lines represent the corresponding products. (A) Phenol. (B) 2,4-Dimethylphenol. The AUCs of the two major peaks are 608 and 676, respectively. (C) 3,4-Dimethylphenol. Docking experiments indicated that the C-6 atom of the compound will be hydroxylated by the enzyme. (D) 2,3,5-Trimethylphenol. The AUCs of the two major peaks are 582 and 262, respectively.
Kinetic characterization of wild-type AoTyr and variants.
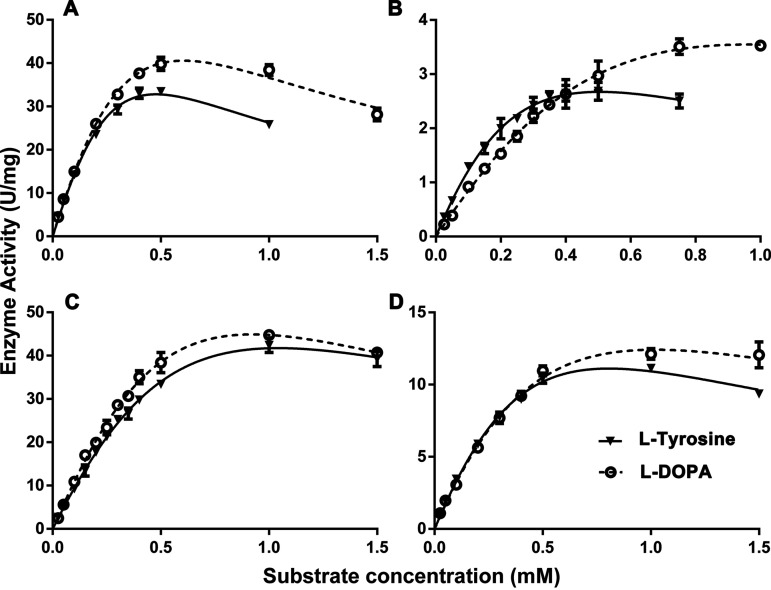
The plot of AoTyr activities versus different concentrations of l-tyrosine and l-DOPA showed obvious substrate inhibitions (Fig. 6A). At substrate levels below 0.5 mM for l-tyrosine and l-DOPA, AoTyr activity obeyed Michaelis-Menten kinetics. Both monophenolase and diphenolase activities increased with increasing substrate concentrations until they reached their highest velocity. In contrast, further increasing the substrate concentration reduced AoTyr activities, indicating that the substrate inhibition effect outweighed the velocity increase. After fitting with the substrate inhibition equation in GraphPad Prism 6, the obtained kinetic parameters toward l-tyrosine and l-DOPA were determined and are listed in Table 2. The kinetic parameters of the monophenolase and diphenolase activities of the wild type (WT) are comparable, and its catalytic efficiencies (kcat/Km ratios) toward l-tyrosine (115 ± 0.39 s−1 mM−1) and l-DOPA (118 ± 0.29 s−1 mM−1) are similar. However, the Ki of the monophenolase activity of the enzyme (0.14 ± 0.06 mM) was significantly lower than that of its diphenolase activity (0.24 ± 0.07 mM).
FIG 6.
Substrate inhibition fitting curve of AoTyr (A) and the D262N (B), D266N (C), and D262N/D266N (D) variants on l-tyrosine and l-DOPA.
TABLE 2.
Kinetic parameters of AoTyr and variants
| Variant | Substrate | Mean value of parameter ± SD |
||||
|---|---|---|---|---|---|---|
| Vmax (U/mg) | Km (mM) | kcat (s−1) | kcat/Km ratio | Ki (mM) | ||
| WT | l-Tyrosine | 257 ± 89.7 | 1.63 ± 0.63 | 188 ± 0.35 | 115 ± 0.39 | 0.14 ± 0.06 |
| l-DOPA | 243 ± 62.2 | 1.51 ± 0.44 | 178 ± 0.26 | 118 ± 0.29 | 0.24 ± 0.07 | |
| D262N | l-Tyrosine | 9.99 ± 2.65 | 0.69 ± 0.22 | 7.32 ± 0.27 | 10.6 ± 0.32 | 0.37 ± 0.15 |
| l-DOPA | 20.6 ± 10.9 | 2.31 ± 1.34 | 15.1 ± 0.53 | 6.54 ± 0.58 | 0.40 ± 0.28 | |
| D266N | l-Tyrosine | 216 ± 51.0 | 2.18 ± 0.58 | 159 ± 0.24 | 72.7 ± 0.27 | 0.50 ± 0.15 |
| l-DOPA | 304 ± 100 | 2.66 ± 0.96 | 223 ± 0.33 | 83.7 ± 0.36 | 0.32 ± 0.13 | |
| D262N/D266N | l-Tyrosine | 48.2 ± 8.90 | 1.35 ± 0.29 | 35.4 ± 0.19 | 26.2 ± 0.21 | 0.48 ± 0.12 |
| l-DOPA | 40.6 ± 8.68 | 1.16 ± 0.30 | 29.8 ± 0.22 | 25.7 ± 0.26 | 0. 90 ± 0.31 | |
The roles of D262 and D266 in substrate recognition and activity modulation were investigated by site-directed mutagenesis. Three variants (D262N, D266N, and D262N/D266N) were constructed using the primers listed in Table 1. Similar to the WT, the plot of activities against substrate concentrations indicated the existence of substrate inhibition in all variants (Fig. 6B to D). However, the Ki values for both monophenolase and diphenolase activities in all variants are significantly decreased compared to those of the WT (Table 2). Compared to the WT, the Ki values for l-tyrosine and l-DOPA in the D262N variant increased by 2.4-fold and 1.67-fold, respectively. The mutation at D266 caused 3.6-fold and 1.33-fold increases in the Ki values for l-tyrosine and l-DOPA, respectively. The largest change in the Ki is observed in the dually mutated variant D262N/D266N, for which the Ki values for l-DOPA and l-tyrosine increased 4-fold and 3.4-fold, respectively.
TABLE 1.
Primers used for AoTyr gene cloning and site-directed mutagenesis
| Variant primer | Sequence (5′–3′)a |
|---|---|
| WT-F | CGACGGATCCATGTCTCGCTTCATCATTAC |
| WT-R | CAGCTCGAGTCACTCAGGTTGACTACCAC |
| D262N-F | CGTTAGAGGCGATCCATAATGGTATTCACGATGATATTGGAGC |
| D262N-R | CAGCTCCAATATCATCGTGAATACCATTATGGATCGCCTC |
| D266N-F | CGTTAGAGGCGATCCATGATGGTATTCACAATGATATTGGAGC |
| D266N-R | CAGCTCCAATATCATTGTGAATACCATCATGGATCGCCTC |
| D262N/D266N-F | GTAACTCGTTAGAGGCGATCCATAATGGTATTCACAATG |
| D262N/D266N-R | CTCCAATATCATTGTGAATACCATTATGGATCGCCTCTAAC |
The underlining in the sequences of the primer pair WT-F and -R indicates the BamHI and XhoI restriction sites, respectively. The mutated bases are marked in boldface type.
The kinetic parameters of the three variants were also measured (Table 2). The results showed that the Km of the D262N variant toward l-tyrosine (0.69 ± 0.22 mM) is significantly decreased compared to that of the WT, whereas the Km of the variant toward l-DOPA is increased (2.31 ± 1.34 mM). As for D266N, its Km values toward both l-tyrosine (2.18 ± 0.58 mM) and l-DOPA (2.66 ± 0.96 mM) are increased compared to those of the WT. In contrast to D266N, the Km values of the dually mutated variant D262N/D266N toward l-tyrosine (1.35 ± 0.29 mM) and l-DOPA (1.16 ± 0.30 mM) are decreased. The catalytic efficiencies of all variants, represented by the kcat/Km ratio, are significantly decreased compared to the WT, and the D262N variant has the lowest values toward both l-tyrosine (10.6 ± 0.32 s−1) and l-DOPA (6.54 ± 0.58 s−1). However, the monophenolase/diphenolase catalytic efficiency ratio for D262N is 1.63, which is significantly higher than that of the WT (0.98). In contrast, the ratios for D266N (0.87) and D262N/D266N (1.02) are similar to that of the WT.
DISCUSSION
In the present work, we report the cloning and heterologous expression of a novel tyrosinase gene from Armillaria ostoyae as well as the purification and characterization of the enzyme. Initially, the putative tyrosinase gene (AoTyr) was cloned using a primer pair based on the coding sequence under accession number SJL09928.1 in the GenBank database. However, to our surprise, the DNA sequencing results showed that the sequence of AoTyr is not identical to the gene sequence that we expected. It has an identity of 97.4% with the encoded sequence under accession number SJL09928.1, and the amino acid sequence of AoTyr has an identity of 96.9% with the sequence under accession number SJL09928.1. Internal transcribed spacer (ITS) (41) sequence PCR was performed, and the obtained ITS1 and ITS4 sequences clearly demonstrated that the strain is Armillaria ostoyae (data not shown).
So far, many tyrosinases have been characterized regarding their structural and functional properties (25), and these comprehensive enzyme data are available at the BRENDA server (https://www.brenda-enzymes.org/enzyme.php?ecno=1.14.18.1) (42). In general, the structure of fungal tyrosinase is more similar to the structure of plant tyrosinase than to that of the bacterial tyrosinase. Both plant and fungal tyrosinases are expressed in a latent form initially, which can be activated through a variety of treatments or agents (43), including biochemical reactions such as hydrolysis (44), pH shock (45, 46), urea (47), detergents such as SDS (48), and fatty acids (49). Similarly, latent AoTyr also has an N-terminal catalytic domain and a C-terminal extension domain, and the C-terminal domain blocks the access of substrate to the active site, maintaining the inactivation of the enzyme (50).
To characterize AoTyr, the full-length enzyme was heterologously expressed in an E. coli system. Expression trials yielded only inclusion bodies, but we were inspired by reports of soluble expression using GroEL chaperones (51, 52) and finally obtained soluble AoTyr. Activity assays showed that the obtained latent AoTyr has no enzymatic activity (data not shown). This is due to the presence of the C-terminal extension domain, which is not involved in the enzyme activity but is essential for the correct folding of the N-terminal domain (53). The removal of the C-terminal domain or the presence of detergents could induce changes in the orientation of the substrate entrance region, leading to the activation of the enzyme (50, 54). Therefore, two strategies were tried to activate latent AoTyr: one is proteolysis by trypsin and another is treatment with different concentrations of SDS. For trypsin activation, the cleavage site was measured to be at R407, similar to the tyrosinase from Polyporus arcularius, which has a cleavage site at R388 (51). The activation of latent AoTyr by SDS was achieved at a minimum concentration of 0.5 mM (Fig. 3B), similar to the optimal SDS concentration (0.6 mM) for AbPPO4 activation (30). The inhibitory effect of high concentrations of SDS may be caused by the denaturation nature of the detergent on the enzyme catalytic domain.
The pH profiles of the monophenolase and diphenolase activities of AoTyr are almost the same, and they are similar to those of the psychrophilic tyrosinase from the marine archaeon “Candidatus Nitrosopumilus koreensis” (tyrosinase-CNK) (28). Both of them have an optimal pH of 6, which is lower than that of AbPPO4 (30) but slightly higher than that of NP_518458 (24). The relatively low optimal pH makes AoTyr a good candidate for the synthesis of catechols, which are unstable at relatively high pHs and temperatures (55). The optimal temperature for AoTyr is ∼25°C (Fig. 3C), which is much lower than the temperature used for NP_518458 (37°C) (24) but similar to those used for several fungal and plant tyrosinases (56). AoTyr became almost inactive at 10°C, while it maintained half of its highest activity as the temperature went to 60°C. This may due to the higher stability of its structure than those of psychrophilic enzymes such as tyrosinase-CNK (28). The relatively high-temperature tolerance of AoTyr makes it suitable for application in processes that require higher temperatures such as 40°C.
Organic solvent tolerance is an important property of enzymes developed for biotechnology applications, especially for tyrosinase, whose substrates are phenolics and insoluble in water. AoTyr shows significant tolerance toward acetone but is sensitive to methanol and DMSO, indicating the compatibility of acetone with the activity of the enzyme (57). This is different from many other tyrosinases such as SPRTyr and CZA14Tyr, which are more sensitive to acetone (58).
As for all tested chemicals (Fig. 4B), AoTyr is directly inactivated by reducing reagents, which may due to the reduction of the binuclear copper at the active site of the enzyme (59). Besides, AoTyr retained >80% residual activity in the presence of 1 mM or 0.1% K+, Li+, Ca2+, Ba2+, or Triton X-100, and its ability to retain >100% activity in the presence of Fe2+, Mn2+, Mg2+, Al3+, urea, SDS, phenylmethylsulfonyl fluoride (PMSF), and Tween 20 indicates its potential for application in industries such as biosensors. Other metal ions such as Cu2+, Zn2+, Ag+, and Co2+ significantly inhibited the activity, suggesting the interruption of these ions to the coordinated copper atoms. This is different from SPRTyr and CZA14Tyr, whose activities are suppressed by Cu2+ only (58). Interestingly, AoTyr showed a relatively high tolerance to EDTA at a 1 mM concentration compared to SPRTyr and CZA14Tyr (58), indicating the relatively higher binding affinity of copper ions for the enzyme.
Unlike most reported tyrosinases (24), the monophenolase activity of AoTyr is comparable to its diphenolase activity (Table 2), which makes this enzyme a potential candidate to be developed for catechol biosynthesis. The substrate specificity of the enzyme toward different monophenols has been tested using HPLC, and the results indicated that the enzyme can act on all tested monophenols (Fig. 5). Interestingly, only a single dominant peak for benzoquinones was observed in the product HPLC spectra when using phenol and 3,4-dimethylphenol as the substrates (Fig. 5A and C), whereas the peaks for both catechols and benzoquinones existed when 2,4-dimethylphenol and 2,3,5-trimethylphenol were used as the substrates (Fig. 5B and D). In addition, the AUC ratio of catechol/benzoquinone in the product of 2,4-dimethylphenol is 1.11, which is more than twice the ratio of the product of 2,3,5-trimethylphenol (0.45). These results indicated that with a reduced reaction time or a decreased quantity of the enzyme in the reaction, the catechol intermediates may become the predominant products. Furthermore, the catalytic activities of AoTyr on l- and d-tyrosine are significantly different. The conversion rate for l-tyrosine is 2.54 times higher than the rate for the d-enantiomer, similar to AbPPO4, which has a specific activity on l-tyrosine 2.58-fold higher than that on d-tyrosine (30).
Interestingly, activity assays showed that both the monophenolase and diphenolase activities of AoTyr suffer substrate inhibition by l-tyrosine and l-DOPA, respectively (Fig. 6). So far, there are several reports describing substrate inhibition in tyrosinase (60–62), and different theories, such as allosteric inhibition (63) and alternative binding sites (64), have been proposed to explain the underlying mechanisms. However, little attention has been paid to the substrate recognition and binding residues that have been proven to play important roles in substrate inhibition of enzymes (65). In the present study, molecular docking was employed to identify key residues that may be involved in the substrate inhibition of AoTyr. This analysis suggests that residues D262 and D266 participate in the binding of l-tyrosine through hydrogen bond interactions and π-anion interactions. Besides, previous reports have shown that residues N205 and R209 (equivalent to residues D262 and D266, respectively, in AoTyr) in TyrBm play important roles in the activities of TyrBm (32, 39). Therefore, the roles of D262 and D266 in the substrate recognition and activity modulation of AoTyr were investigated by mutagenesis experiments.
The results from enzyme activity assays showed that the substrate inhibition phenomenon still exists in all variants (D262N, D266N, and D262N/D266N) (Fig. 6). The replacement of residue D262 by a neutral asparagine resulted in a dramatic decrease in both monophenolase and diphenolase activities (Table 2). The Vmax of the variant for l-tyrosine (9.99 ± 2.65 U/mg) is almost half of the value for l-DOPA (20.6 ± 10.9 U/mg). However, the kcat/Km ratio of the variant for l-tyrosine (10.6 ± 0.32 s−1 mM−1) is significantly higher than that for l-DOPA (6.54 ± 0.58 s−1 mM−1), indicating the important role that the residue plays in the modulation of the catalytic efficiency of the monophenolase and diphenolase activities of the enzyme. Moreover, its Ki values for both substrates are significantly increased compared to those of the WT, indicating that the negative charge of D262 plays an important role in the substrate inhibition of the enzyme. As for residue D266, the replacement with asparagine also led to decreased monophenolase and diphenolase activities. In contrast to the D262N variant, the kcat/Km ratio of D266N for l-tyrosine (72.7 ± 0.27 s−1 mM−1) is lower than that for l-DOPA (83.7 ± 0.36 s−1 mM−1), indicating the countereffect role of the residue compared to D262N in the modulation of the catalytic efficiency of the monophenolase and diphenolase activities of the enzyme. Besides, the Ki values of both monophenolase and diphenolase activities of the enzyme are also increased, and the Ki for l-tyrosine is more than 3.5 times higher than that of the WT. This suggests that the negative charge of D266 also plays an important role in the substrate inhibition of the enzyme, similar to D262. Simultaneous mutation at both sites also led to significant decreases in the enzymatic activities for both substrates. Different from the single-site variants, the kcat/Km ratios of the D262N/D266N variant for l-tyrosine and l-DOPA are similar, which substantiates the opposite roles of the two residues in the catalytic efficiencies of the monophenolase and diphenolase activities of the enzyme. Moreover, the Ki for l-tyrosine of the D262N/D266N variant is similar to that of D266N, whereas its Ki for l-DOPA is 3.75-fold higher than that of the WT. These results demonstrated that the replacement of D262 and D266 with neutral asparagine significantly modulates the monophenolase/diphenolase activities and substrate inhibition of AoTyr.
MATERIALS AND METHODS
Materials.
The fungus A. ostoyae was cultured in our laboratory. E. coli DH5α competent cells were obtained from Novagen (USA), while E. coli BL21(DE3)/pGro7 competent cells were purchased from Nonoprotein (China). The vectors pMD19-T and pET28a were obtained from TaKaRa Biotechnology (China) and Invitrogen (USA), respectively. Enzymes and reagents related to PCR and DNA manipulation were purchased from TaKaRa Biotechnology (China). The different substrates were obtained from Sigma and Alfa Aesar. All reagents were of analytical grade. All curve fitting was performed using GraphPad Prism version 7 for Windows (GraphPad Software, La Jolla, CA, USA).
Gene cloning and variant construction of A. ostoyae tyrosinase.
The total RNA of A. ostoyae was extracted using the spin column plant total RNA purification kit from Sangon Biotech (China) according to the manual’s instructions. The obtained RNA was subsequently used for cDNA synthesis with the RevertAid first-strand cDNA synthesis kit (Thermo Fisher Scientific, China). The full-length open reading frame of the A. ostoyae tyrosinase gene (AoTyr) was PCR amplified from the cDNA template with the primer pairs WT-F and WT-R (Table 1) based on the annotated tyrosinase (GenBank accession number SJL09928.1)-encoding gene. The PCR product was directly ligated into the linearized vector pMD19-T through TA cloning. After blue/white screening, the recombinant plasmid pMD19-AoTyr in a positive colony was extracted and sent to BGI (China) for sequencing. At the same time, the AoTyr gene in the pMD19-AoTyr plasmid was cut off by double digestion using BamHI and XhoI, which was then ligated into the BamHI-XhoI sites of pET28a for expression. The obtained recombinant plasmid was transformed into DH5α cells, and the integrity of the recovered plasmid was confirmed by PCR using the primers described above and double digestion using BamHI and XhoI. Based on the obtained pMD19-AoTyr plasmid, variants were generated by restriction-free (RF) cloning (66) using the primers listed in Table 1.
In silico analysis.
Primary amino acid sequence analysis was performed by using BLASTP (https://blast.ncbi.nlm.nih.gov/Blast.cgi) against the RefSeq protein database of the NCBI, and a multisequence alignment of AoTyr with several fungal tyrosinases was performed using ESPript 3.0 (67). To further investigate the structural characteristic of AoTyr, the 3D structure of the enzyme was constructed by homology modeling using the SWISS-MODEL online sever (37). The crystal structure of AbPPO4 (PDB accession number 5M6B) (30) was used as the template since it shares the highest sequence identity (44.39%) with AoTyr. The docking study was performed using the Tripos Sybyl X-2.1.1 software package. The model of l-tyrosine for the docking study was downloaded from the PDB database (https://www.rcsb.org/ligand/TYR). The protein structure was initially energetically minimized using the Tripos force field (68) with default parameters. The Surflex-Dock (SFXC) docking mode was employed for molecular docking calculation, and the binding pocket was defined through automatic assignment with default parameters followed by manual inspection. The ligand-protein interactions were visualized and analyzed using UCSF Chimera software (69).
Protein expression and purification.
Competent E. coli BL21(DE3)/pGro7 cells were transformed with the obtained recombinant WT pET28a-AoTyr plasmid and its variants. A positive colony was picked and inoculated in 50 ml of Luria-Bertani (LB) medium supplied with 50 μg/ml kanamycin and 34 μg/ml chloramphenicol at 37°C overnight with constant shaking. Next, the stock cells were added into fresh LB medium at a ratio of 1:100 (vol/vol), which was supplied with the appropriate antibiotics and 0.2 mM CuSO4, with constant shaking. When the optical density at 600 nm (OD600) reached 0.6, the coexpression of AoTyr and GroEL/ES chaperones was induced with the addition of isopropyl-β-d-1-thiogalactopyranoside (IPTG) and l-arabinose to final concentrations of 0.02 mM and 10 mM, respectively. The medium was further cultured at 20°C for 24 h before being harvested by centrifugation at 8,000 rpm for 10 min. The obtained cell pellets were stored at −80°C until use.
All purifications were carried out at 4°C unless otherwise stated. The cell pellets were resuspended in lysis buffer (40 mM Tris [pH 8.0], 100 mM NaCl, 10% [vol/vol] glycerol, 10 mM imidazole, 1 mM phenylmethylsulfonyl fluoride [PMSF]). The resulting slurry was sonicated on ice, and the crude extract was centrifuged at 16,000 × g for 30 min. The supernatant was loaded onto a Ni-Sepharose 6 Fast Flow column (GE Healthcare) preequilibrated with binding buffer (40 mM Tris [pH 8.0], 100 mM NaCl, 10% [vol/vol] glycerol, and 10 mM imidazole). The target protein was eluted by a stepwise imidazole gradient with increasing concentrations in binding buffer. The eluted fractions were analyzed by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) to monitor the purity. The fraction with high purity was kept, and the solution buffer was changed to buffer A (40 mM Tris [pH 7.5], 20% [vol/vol] glycerol) by repeated concentration and dilution with a 30-kDa-molecular-weight-cutoff Amicon ultracentrifugal filter unit (EMD Millipore, China). Protein concentrations were determined by the Bradford assay (70), from the absorption at 595 nm, using bovine serum albumin as a standard.
Activation of latent tyrosinase.
The proteolytic activation of latent AoTyr was carried out with trypsin. The concentration of purified latent AoTyr was adjusted to 1 mg/ml in buffer A, in which trypsin was added to a final concentration of 50 mg/ml. The treatment was performed at 4°C for 1 h and terminated by adding PMSF to a final concentration of 2 mM. Activated AoTyr was further purified using a HiTrap Q FF anion-exchange chromatography column (GE Life Sciences, USA). The fractions with tyrosinase activity were pooled and concentrated. The final concentration was measured by the Bradford assay. The activation of latent AoTyr by SDS was carried out in the presence of various concentrations of SDS (0.05, 0.1, 0.5, 1, 5, and 10 mM) during the standard reaction.
Tyrosinase activity assays.
The monophenolase and diphenolase activities of AoTyr and variants were determined spectrophotometrically using l-tyrosine and l-3,4-dihydroxyphenylalanine (l-DOPA) as the substrates, respectively. The standard reaction mixtures (total volume of 0.5 ml) contained 0.3 mM substrate and 100 mM sodium phosphate buffer (pH 6.0). The reactions were initiated by adding 2.5 μl of the enzyme at the appropriate concentration, and the mixtures were incubated using Eppendorf ThermoMixer C with a mixing speed of 700 rpm at 25°C. The reactions were finished in 10 min, and the formation of l-dopachrome was recorded at 475 nm (ε475 = 3,600 M−1 cm−1) (28). One unit of enzyme activity was defined as the amount of enzyme required to produce 1 μmol product from the appropriate substrate per min under the assay conditions. All measurements were carried out in triplicate. The kinetic parameters of AoTyr were determined using different concentrations of the substrate, including 0.025, 0.05, 0.1, 0.2, 0.3, 0.4, 0.5, 0.75, 1, and 1.5 mM. The kinetic properties were calculated by fitting the measured activities to the Michaelis-Menten equation or the substrate inhibition equation by nonlinear regression analysis implemented in GraphPad Prism 6.
Effects of temperature and pH on AoTyr activity.
The effect of temperature on AoTyr was determined with reactions carried out in 20 mM sodium phosphate buffer (pH 5.0) using 1 mM l-DOPA as the substrate with different temperatures ranging from 0°C to 80°C. The monophenolase and diphenolase activities of AoTyr at different pHs were determined by an l-dopachrome formation assay using l-tyrosine and l-DOPA as the substrates, respectively, at concentrations of 0.3 mM. Buffers used for different pH values included 100 mM sodium acetate (pH 3 to 5), 100 mM sodium phosphate buffer (pH 6 to 8), 100 mM Tris-HCl (pH 9), and 100 mM 3-(cyclohexylamino)-1-propanesulfonic acid (CAPS) (pH 10 and 11).
Effect of organic solvents on AoTyr activity.
Several different organic solvents such as methanol, ethanol, acetone, and DMSO were used to determine their effects on AoTyr activity. The residual activities were measured by an l-dopachrome formation assay using 1 mM l-DOPA as the substrate. The reaction mixtures contained different solvents to a final concentration of 30% (vol/vol) and 20 mM sodium phosphate buffer (pH 5.0). The reactions were carried out at 25°C and lasted for 1 h.
Effect of metal ions, detergents, and inhibitors on AoTyr activity.
The l-dopachrome release assay was used to determine the effect of metal ions, detergents, and inhibitors on AoTyr activity using 1 mM l-DOPA as the substrate. The reaction buffer contained 20 mM sodium phosphate buffer (pH 5.0) and was preloaded with different chemicals at the desired final concentrations. Metal ions used in the assay included K+, Li+, Ag+, Zn2+, Ba2+, Ca2+, Co2+, Cu2+, Fe2+, Mg2+, Mn2+, and Al3+ at a final concentration of 1 mM. Detergents and inhibitors used in the experiment included urea, SDS, EDTA, PMSF, β-ME, DTT, Tween 20, and Triton X-100 at a final concentration of 0.1% (vol/vol) or 1 mM. The reactions were carried out at 25°C and lasted for 1 h.
Stereoselectivity and substrate specificity.
The stereoselectivity of AoTyr was investigated under standard conditions using l-tyrosine and d-tyrosine as the substrates. For the determination of the monophenolase activity of AoTyr toward different substrates, several monophenols, including phenol, 2,4-dimethylphenol, 3,4-dimethylphenol, and 2,3,5-trimethylphenol, were used as the substrates. The reaction buffer was 20 mM sodium phosphate (pH 5.0). The reactions were carried out at 25°C and lasted for 24 h. The products in the reaction mixtures were analyzed by HPLC on a BDS Hypersil C18 4.6- by 250-mm column (Thermo Scientific) with a mobile phase of 30% (vol/vol) acetonitrile in 0.025% (vol/vol) phosphoric acid. The UV-visible (UV-Vis) detector was set to 270 nm, the flow rate was 1 ml/min, and the injection volume was 20 μl.
Data availability.
AoTyr sequence data were deposited in GenBank under accession number MW773195.
ACKNOWLEDGMENTS
This work was supported by a Chinese National Natural Science Foundation grant (31770847) and the Dalian Science and Technology Innovation Fund-Key & Major Subject (2020JJ25CY017). Tang Li was supported by the Doctoral Scientific Research Foundation of Liaoning Province (2020-BS-013). Heng Yin was supported by the LiaoNing Revitalization Talents Program (XLYC1807041) and the Outstanding Member Fund of the CAS Youth Innovation Promotion Association (Y201939).
We declare that we have no conflict of interest.
Contributor Information
Heng Yin, Email: yinheng@dicp.ac.cn.
Emma R. Master, University of Toronto
REFERENCES
- 1.Goldfeder M, Egozy M, Shuster Ben-Yosef V, Adir N, Fishman A. 2013. Changes in tyrosinase specificity by ionic liquids and sodium dodecyl sulfate. Appl Microbiol Biotechnol 97:1953–1961. 10.1007/s00253-012-4050-z. [DOI] [PubMed] [Google Scholar]
- 2.Eswaramoorthy S, Bonanno JB, Burley SK, Swaminathan S. 2006. Mechanism of action of a flavin-containing monooxygenase. Proc Natl Acad Sci U S A 103:9832–9837. 10.1073/pnas.0602398103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meunier B, de Visser SP, Shaik S. 2004. Mechanism of oxidation reactions catalyzed by cytochrome p450 enzymes. Chem Rev 104:3947–3980. 10.1021/cr020443g. [DOI] [PubMed] [Google Scholar]
- 4.van Gelder CW, Flurkey WH, Wichers HJ. 1997. Sequence and structural features of plant and fungal tyrosinases. Phytochemistry 45:1309–1323. 10.1016/s0031-9422(97)00186-6. [DOI] [PubMed] [Google Scholar]
- 5.Claus H, Decker H. 2006. Bacterial tyrosinases. Syst Appl Microbiol 29:3–14. 10.1016/j.syapm.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Mayer AM. 2006. Polyphenol oxidases in plants and fungi: going places? A review. Phytochemistry 67:2318–2331. 10.1016/j.phytochem.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Halaouli S, Asther M, Sigoillot JC, Hamdi M, Lomascolo A. 2006. Fungal tyrosinases: new prospects in molecular characteristics, bioengineering and biotechnological applications. J Appl Microbiol 100:219–232. 10.1111/j.1365-2672.2006.02866.x. [DOI] [PubMed] [Google Scholar]
- 8.Marusek CM, Trobaugh NM, Flurkey WH, Inlow JK. 2006. Comparative analysis of polyphenol oxidase from plant and fungal species. J Inorg Biochem 100:108–123. 10.1016/j.jinorgbio.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Riley PA. 1997. Molecules in focus: melanin. Int J Biochem Cell Biol 29:1235–1239. 10.1016/S1357-2725(97)00013-7. [DOI] [PubMed] [Google Scholar]
- 10.Cerenius L, Soderhall K. 2004. The prophenoloxidase-activating system in invertebrates. Immunol Rev 198:116–126. 10.1111/j.0105-2896.2004.00116.x. [DOI] [PubMed] [Google Scholar]
- 11.Muller WEG, Grebenjuk VA, Thakur NL, Thakur AN, Batel R, Krasko A, Muller IM, Breter HJ. 2004. Oxygen-controlled bacterial growth in the sponge Suberites domuncula: toward a molecular understanding of the symbiotic relationships between sponge and bacteria. Appl Environ Microbiol 70:2332–2341. 10.1128/aem.70.4.2332-2341.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorman MJ, Arakane Y. 2010. Tyrosine hydroxylase is required for cuticle sclerotization and pigmentation in Tribolium castaneum. Insect Biochem Mol Biol 40:267–273. 10.1016/j.ibmb.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taft AS, Chen CC, Li J, Christensen BM. 2001. Molecular cloning of two prophenoloxidase genes from the mosquito Aedes aegypti. Insect Mol Biol 10:97–103. 10.1046/j.1365-2583.2001.00242.x. [DOI] [PubMed] [Google Scholar]
- 14.Soler-Rivas C, Jolivet S, Arpin N, Olivier JM, Wichers HJ. 1999. Biochemical and physiological aspects of brown blotch disease of Agaricus bisporus. FEMS Microbiol Rev 23:591–614. 10.1111/j.1574-6976.1999.tb00415.x. [DOI] [PubMed] [Google Scholar]
- 15.Quideau S, Pouysegu L. 1999. Synthetic uses of orthoquinone monoketals and their orthoquinol variants. A review. Org Prep Proced Int 31:617–680. 10.1080/00304949909355348. [DOI] [Google Scholar]
- 16.Nair V, Kumar S. 1994. Hetero diels-alder reactions of 3,5-di-tert-butyl-o-benzoquinone with acyclic dienes: novel synthesis of 1,4-benzodioxines. J Chem Soc Chem Commun 1994:1341–1342. 10.1039/C39940001341. [DOI] [Google Scholar]
- 17.Agarwal P, Singh M, Singh J, Singh RP. 2019. Microbial tyrosinases: a novel enzyme, structural features, and applications, p 3–19. In Shukla P (ed), Applied microbiology and bioengineering: an interdisciplinary approach. Academic Press, London, United Kingdom. 10.1016/b978-0-12-815407-6.00001-0. [DOI] [Google Scholar]
- 18.Michalik J, Emilianowicz-Czerska W, Switalski L, Raczyńska-Bojanowska K. 1975. Monophenol monooxygenase and lincomysin biosynthesis in Streptomyces lincolnensis. Antimicrob Agents Chemother 8:526–531. 10.1128/aac.8.5.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirk KL, Creveling CR. 1984. The chemistry and biology of ring-fluorinated biogenic amines. Med Res Rev 4:189–220. 10.1002/med.2610040204. [DOI] [PubMed] [Google Scholar]
- 20.Liu N, Zhang T, Wang YJ, Huang YP, Ou JH, Shen P. 2004. A heat inducible tyrosinase with distinct properties from Bacillus thuringiensis. Lett Appl Microbiol 39:407–412. 10.1111/j.1472-765X.2004.01599.x. [DOI] [PubMed] [Google Scholar]
- 21.Surwase SN, Jadhav JP. 2011. Bioconversion of L-tyrosine to L-DOPA by a novel bacterium Bacillus sp. JPJ. Amino Acids 41:495–506. 10.1007/s00726-010-0768-z. [DOI] [PubMed] [Google Scholar]
- 22.Yabuki C, Yagi K, Nanjo F. 2017. Highly efficient synthesis of theaflavins by tyrosinase from mushroom and its application to theaflavin related compounds. Process Biochem 55:61–69. 10.1016/j.procbio.2017.02.002. [DOI] [Google Scholar]
- 23.Hansen TV, Skattebøl L. 2005. One-pot synthesis of substituted catechols from the corresponding phenols. Tetrahedron Lett 46:3357–3358. 10.1016/j.tetlet.2005.03.082. [DOI] [Google Scholar]
- 24.Hernandez-Romero D, Sanchez-Amat A, Solano F. 2006. A tyrosinase with an abnormally high tyrosine hydroxylase/dopa oxidase ratio. FEBS J 273:257–270. 10.1111/j.1742-4658.2005.05038.x. [DOI] [PubMed] [Google Scholar]
- 25.Fairhead M, Thony-Meyer L. 2012. Bacterial tyrosinases: old enzymes with new relevance to biotechnology. N Biotechnol 29:183–191. 10.1016/j.nbt.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Zhou YZ, Alany RG, Chuang V, Wen J. 2012. Studies of the rate constant of l-DOPA oxidation and decarboxylation by HPLC. Chromatographia 75:597–606. 10.1007/s10337-012-2229-1. [DOI] [Google Scholar]
- 27.Goldfeder M, Kanteev M, Adir N, Fishman A. 2013. Influencing the monophenolase/diphenolase activity ratio in tyrosinase. Biochim Biophys Acta 1834:629–633. 10.1016/j.bbapap.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 28.Kim H, Yeon YJ, Choi YR, Song W, Pack SP, Choi YS. 2016. A cold-adapted tyrosinase with an abnormally high monophenolase/diphenolase activity ratio originating from the marine archaeon Candidatus Nitrosopumilus koreensis. Biotechnol Lett 38:1535–1542. 10.1007/s10529-016-2125-0. [DOI] [PubMed] [Google Scholar]
- 29.Wu J, Chen H, Gao J, Liu X, Cheng W, Ma X. 2010. Cloning, characterization and expression of two new polyphenol oxidase cDNAs from Agaricus bisporus. Biotechnol Lett 32:1439–1447. 10.1007/s10529-010-0329-2. [DOI] [PubMed] [Google Scholar]
- 30.Pretzler M, Bijelic A, Rompel A. 2017. Heterologous expression and characterization of functional mushroom tyrosinase (AbPPO4). Sci Rep 7:1810. 10.1038/s41598-017-01813-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawamura-Konishi Y, Tsuji M, Hatana S, Asanuma M, Kakuta D, Kawano T, Mukouyama EB, Goto H, Suzuki H. 2007. Purification, characterization, and molecular cloning of tyrosinase from Pholiota nameko. Biosci Biotechnol Biochem 71:1752–1760. 10.1271/bbb.70171. [DOI] [PubMed] [Google Scholar]
- 32.Goldfeder M, Kanteev M, Isaschar-Ovdat S, Adir N, Fishman A. 2014. Determination of tyrosinase substrate-binding modes reveals mechanistic differences between type-3 copper proteins. Nat Commun 5:4505. 10.1038/ncomms5505. [DOI] [PubMed] [Google Scholar]
- 33.Mauracher SG, Molitor C, Al-Oweini R, Kortz U, Rompel A. 2014. Latent and active abPPO4 mushroom tyrosinase cocrystallized with hexatungstotellurate(VI) in a single crystal. Acta Crystallogr D Biol Crystallogr 70:2301–2315. 10.1107/S1399004714013777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faccio G, Arvas M, Thony-Meyer L, Saloheimo M. 2013. Experimental and bioinformatic investigation of the proteolytic degradation of the C-terminal domain of a fungal tyrosinase. J Inorg Biochem 121:37–45. 10.1016/j.jinorgbio.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Davis AV, O’Halloran TV. 2008. A place for thioether chemistry in cellular copper ion recognition and trafficking. Nat Chem Biol 4:148–151. 10.1038/nchembio0308-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson NJ, Winge DR. 2010. Copper metallochaperones. Annu Rev Biochem 79:537–562. 10.1146/annurev-biochem-030409-143539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, Heer FT, de Beer TAP, Rempfer C, Bordoli L, Lepore R, Schwede T. 2018. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res 46:W296–W303. 10.1093/nar/gky427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benkert P, Biasini M, Schwede T. 2011. Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics 27:343–350. 10.1093/bioinformatics/btq662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shuster Ben-Yosef V, Sendovski M, Fishman A. 2010. Directed evolution of tyrosinase for enhanced monophenolase/diphenolase activity ratio. Enzyme Microb Technol 47:372–376. 10.1016/j.enzmictec.2010.08.008. [DOI] [Google Scholar]
- 40.Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins MR, Appel RD, Bairoch A. 2005. Protein identification and analysis tools on the ExPASy server, p 571–607. In Walker JM (ed), The proteomics protocols handbook. Humana Press, Totowa, NJ. 10.1385/1592598900. [DOI] [Google Scholar]
- 41.Raja HA, Miller AN, Pearce CJ, Oberlies NH. 2017. Fungal identification using molecular tools: a primer for the natural products research community. J Nat Prod 80:756–770. 10.1021/acs.jnatprod.6b01085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schomburg I, Jeske L, Ulbrich M, Placzek S, Chang A, Schomburg D. 2017. The BRENDA enzyme information system—from a database to an expert system. J Biotechnol 261:194–206. 10.1016/j.jbiotec.2017.04.020. [DOI] [PubMed] [Google Scholar]
- 43.Gandia-Herrero F, Jimenez-Atienzar M, Cabanes J, Garcia-Carmona F, Escribano J. 2005. Evidence for a common regulation in the activation of a polyphenol oxidase by trypsin and sodium dodecyl sulfate. Biol Chem 386:601–607. 10.1515/BC.2005.070. [DOI] [PubMed] [Google Scholar]
- 44.Galindo JD, Peñafiel R, Varon R, Pedreno E, Garcia-Carmona F, Garcia-Cánovas F. 1983. Kinetic study of the activation process of frog epidermis pro-tyrosinase by trypsin. Int J Biochem 15:633–637. 10.1016/0020-711X(83)90187-8. [DOI] [PubMed] [Google Scholar]
- 45.Kenten RH. 1957. Latent phenolase in extracts of broad-bean (Vicia faba L.) leaves. I. Activation by acid and alkali. Biochem J 67:300–307. 10.1042/bj0670300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valero E, García-Carmona F. 1992. pH-induced kinetic co-operativity of a thylakoid-bound polyphenol oxidase. Biochem J 286:623–626. 10.1042/bj2860623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swain T, Mapson LW, Robb DA. 1966. Activation of Vicia faba (L.) tyrosinase as effected by denaturing agents. Phytochemistry 5:469–482. 10.1016/S0031-9422(00)82161-5. [DOI] [Google Scholar]
- 48.Moore BM, Flurkey WH. 1990. Sodium dodecyl sulfate activation of a plant polyphenoloxidase. Effect of sodium dodecyl sulfate on enzymatic and physical characteristics of purified broad bean polyphenoloxidase. J Biol Chem 265:4982–4988. 10.1016/S0021-9258(19)34072-4. [DOI] [PubMed] [Google Scholar]
- 49.Golbeck JH, Cammarata KV. 1981. Spinach thylakoid polyphenol oxidase. Plant Physiol 67:977–984. 10.1104/pp.67.5.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gerdemann C, Eicken C, Galla HJ, Krebs B. 2002. Comparative modeling of the latent form of a plant catechol oxidase using a molluskan hemocyanin structure. J Inorg Biochem 89:155–158. 10.1016/s0162-0134(01)00399-3. [DOI] [PubMed] [Google Scholar]
- 51.Marková E, Kotik M, Křenková A, Man P, Haudecoeur R, Boumendjel A, Hardré R, Mekmouche Y, Courvoisier-Dezord E, Réglier M, Martínková L. 2016. Recombinant tyrosinase from Polyporus arcularius: overproduction in Escherichia coli, characterization, and use in a study of aurones as tyrosinase effectors. J Agric Food Chem 64:2925–2931. 10.1021/acs.jafc.6b00286. [DOI] [PubMed] [Google Scholar]
- 52.Do H, Kang E, Yang B, Cha HJ, Choi YS. 2017. A tyrosinase, mTyr-CNK, that is functionally available as a monophenol monooxygenase. Sci Rep 7:17267. 10.1038/s41598-017-17635-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moe LL, Maekawa S, Kawamura-Konishi Y. 2015. The pro-enzyme C-terminal processing domain of Pholiota nameko tyrosinase is responsible for folding of the N-terminal catalytic domain. Appl Microbiol Biotechnol 99:5499–5510. 10.1007/s00253-015-6597-y. [DOI] [PubMed] [Google Scholar]
- 54.Selles-Marchart S, Casado-Vela J, Bru-Martinez R. 2007. Effect of detergents, trypsin and unsaturated fatty acids on latent loquat fruit polyphenol oxidase: basis for the enzyme’s activity regulation. Arch Biochem Biophys 464:295–305. 10.1016/j.abb.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 55.Anderson TH, Yu J, Estrada A, Hammer MU, Waite JH, Israelachvili JN. 2010. The contribution of DOPA to substrate-peptide adhesion and internal cohesion of mussel-inspired synthetic peptide films. Adv Funct Mater 20:4196–4205. 10.1002/adfm.201000932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Selinheimo E, NiEidhin D, Steffensen C, Nielsen J, Lomascolo A, Halaouli S, Record E, O’Beirne D, Buchert J, Kruus K. 2007. Comparison of the characteristics of fungal and plant tyrosinases. J Biotechnol 130:471–480. 10.1016/j.jbiotec.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 57.Takemori S, Furuya E, Suzuki H, Katagiri M. 1967. Stabilization of enzyme activity by an organic solvent. Nature 215:417–419. 10.1038/215417a0. [DOI] [PubMed] [Google Scholar]
- 58.Le Roes-Hill M, Palmer Z, Rohland J, Kirby BM, Burton SG. 2015. Partial purification and characterisation of two actinomycete tyrosinases and their application in cross-linking reactions. J Mol Catal B Enzym 122:353–364. 10.1016/j.molcatb.2015.10.012. [DOI] [Google Scholar]
- 59.Naish-Byfield S, Cooksey CJ, Riley PA. 1994. Oxidation of monohydric phenol substrates by tyrosinase: effect of dithiothreitol on kinetics. Biochem J 304(Part 1):155–162. 10.1042/bj3040155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Faria RO, Moure VR, Balmant W, de Almeida Amazonas MAL, Krieger N, Mitchell DA. 2007. The tyrosinase produced by Lentinula boryana (Berk. & Mont.) Pegler suffers substrate inhibition by L-DOPA. Food Technol Biotechnol 45:334–340. [Google Scholar]
- 61.Bru R, Sanchez-Ferrer A, García-Carmona F. 1989. Characteristics of tyrosinase in AOT-isooctane reverse micelles. Biotechnol Bioeng 34:304–308. 10.1002/bit.260340305. [DOI] [PubMed] [Google Scholar]
- 62.Pomerantz SH. 1966. The tyrosine hydroxylase activity of mammalian tyrosinase. J Biol Chem 241:161–168. 10.1016/S0021-9258(18)96973-5. [DOI] [PubMed] [Google Scholar]
- 63.Haghbeen K, Khalili MB, Nematpour FS, Gheibi N, Fazli M, Alijanianzadeh M, Jahromi SZ, Sariri R. 2010. Surveying allosteric cooperativity and cooperative inhibition in mushroom tyrosinase. J Food Biochem 34:308–328. 10.1111/j.1745-4514.2009.00280.x. [DOI] [Google Scholar]
- 64.Hassani S, Gharechaei B, Nikfard S, Fazli M, Gheibi N, Hardré R, Legge RL, Haghbeen K. 2018. New insight into the allosteric effect of L-tyrosine on mushroom tyrosinase during L-dopa production. Int J Biol Macromol 114:821–829. 10.1016/j.ijbiomac.2018.03.185. [DOI] [PubMed] [Google Scholar]
- 65.Li T, Stephen P, Zhu DW, Shi R, Lin SX. 2019. Crystal structures of human 17beta-hydroxysteroid dehydrogenase type 1 complexed with estrone and NADP(+) reveal the mechanism of substrate inhibition. FEBS J 286:2155–2166. 10.1111/febs.14784. [DOI] [PubMed] [Google Scholar]
- 66.van den Ent F, Lowe J. 2006. RF cloning: a restriction-free method for inserting target genes into plasmids. J Biochem Biophys Methods 67:67–74. 10.1016/j.jbbm.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 67.Robert X, Gouet P. 2014. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res 42:W320–W324. 10.1093/nar/gku316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Clark M, Cramer RD, van Opdenbosch N. 1989. Validation of the general purpose tripos 5.2 force field. J Comput Chem 10:982–1012. 10.1002/jcc.540100804. [DOI] [Google Scholar]
- 69.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. 2004. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612. 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 70.Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
AoTyr sequence data were deposited in GenBank under accession number MW773195.