Abstract
BACKGROUND AND PURPOSE: The importance of diffusion-weighted imaging (DWI) for delineating acute ischemic lesions has been investigated extensively; however, few studies have investigated the role of DWI in the subacute stage of stroke. Because these lesions tend to appear bright throughout the first days of ischemia, owing to restricted diffusion, we speculated that DWI could also improve the detection of subacute infarcts as compared with conventional and contrast-enhanced MR imaging.
METHODS: Interleaved echo-planar DWI with phase navigation was performed on a 1.5-T MR unit in a consecutive series of 53 patients (mean age, 66 ± 14 years) with suspected recent cerebral ischemia. The interval between onset of clinical symptoms and MR imaging ranged from 1 to 14 days (mean, 6 ± 4 days). Contrast material was given to 28 patients in a dose of 0.1 mmol/kg.
RESULTS: DWI clearly delineated recent ischemic damage in 39 patients (74%) as compared with 33 (62%) in whom lesions were identified or suspected on conventional T2-weighted images. DWI provided information not accessible with T2-weighted imaging in 17 patients when evidence of lesion multiplicity or detection of clinically unrelated recent lesions was included for comparison. Subacute ischemic lesions were also seen more frequently on DWI sequences than on contrast-enhanced images (20 versus 13 patients). DWI was more likely to make a diagnostic contribution in the first week of stroke and in patients with small lesions or preexisting ischemic cerebral damage than was conventional MR imaging.
CONCLUSION: Recent ischemic damage is better shown on DWI sequences than on conventional and contrast-enhanced MR images throughout the first days after stroke and may provide further information about the origin of clinical symptoms. Adding DWI to imaging protocols for patients with subacute cerebral ischemia is recommended.
Diffusion-weighted imaging (DWI) has been shown to contribute significantly to the early detection of acute ischemic infarction and is recognized as a bright lesion because of a drop in diffusivity (1–7). This phenomenon of restricted diffusion associated with ischemic damage persists for at least 4 to 6 days. Thereafter, diffusion starts to increase and hyperintensity on DWI studies begins to vanish. In clinical practice, it frequently is important not just to delineate the extent of ischemic damage within the first hours but to identify the size, number, and location of lesions at a later stage as well. In some instances it may simply be helpful to confirm the diagnosis of ischemic stroke and to clarify the origin of clinical symptoms. Moreover, morphologic data can add information on the pathogenesis of stroke and the prognosis. Although it is generally not difficult to determine the occurrence of territorial infarcts on conventional MR images, owing to the characteristic morphologic features, this may not always be so easy in cases of lacunar infarction. It may be impossible to separate acute small lesions without mass effect from concomitant areas of signal hyperintensity, especially in the presence of preexisting brain damage. In this setting, the application of contrast material has been proposed to be helpful for diagnosis (8–12). However, this adds further costs to a patient's examination, and false-negative results are frequent in the presence of incomplete blood brain–barrier damage. Hence, selective delineation of recent ischemic damage by means of DWI could be an ideal alternative. We therefore set out to test the diagnostic contribution of DWI in the subacute phase of cerebral ischemia.
Methods
With the implementation of a new sequence for high-quality DWI on conventional gradient systems this technique was added routinely to our MR protocol for examination of stroke patients (13). After an enrollment period of 9 months, we analyzed the imaging studies of all patients referred for suspected cerebral ischemia. The interval between onset of acute symptoms and MR examination ranged from 1 to 14 days. Patients with a hematoma or other nonischemic gross morphologic abnormalities were excluded. The remaining study population consisted of 53 patients (21 women, 32 men; mean age, 66 ± 14 years; range, 30–87 years).
MR imaging was performed on a 1.5-T whole-body unit. For DWI, we used an interleaved echo-planar data acquisition technique with phase navigation to correct for bulk motion. Cardiac gating based on the plethysmographic waveform from a finger-pulse oximeter served to minimize influences of pulsatile brain motion. Fat suppression was established by a spectral presaturation pulse. We used two levels of diffusion weighting: one with only slight diffusion weighting, which renders the diffusion contrast more or less T2-weighted, and the other with large b values (b = 716 s/mm2), in which the diffusion-sensitizing gradients were placed in a phase-encode direction. A more detailed description of this technique has been provided elsewhere (13).
Conventional MR imaging consisted of a dual spin-echo (SE) sequence in 25 patients (2500/30,90 [TR/TE]) and a fast spin-echo (FSE) sequence in 28 patients (2900/120). In addition, a fast CSF-suppressed T2-weighted SE sequence (fluid-attenuated inversion recovery [FLAIR]) was obtained in 39 patients (6000/130, TI = 1900). Contrast-enhanced studies were obtained in 28 patients in a dose of 0.1 mmol/kg body weight. The indications for administration of contrast material were either unclear or negative findings in the noncontrast series. The section thickness for all sequences was 5 mm, with a gap of 0.5 to 1.5 mm.
First, all the patients' studies in a given sequence were randomly mixed and the DWI, T2-weighted, and contrast-enhanced images were interpreted separately for presence, location, and number of acute ischemic lesions, with the reviewers blinded to the patients' symptoms. The set of T2-weighted images always included either a proton density–weighted or FLAIR sequence. Thereafter, the set of T2-weighted images was searched for subacute lesions with all clinical information provided. A third step comprised side-to-side comparisons of all sequences. The severity of stroke symptoms was graded as mild, moderate, or severe, in accordance with the Graded Neurologic Scale (14). Symptom duration of less than 24 hours was interpreted as a transient ischemic attack (TIA).
Results
At the time of MR imaging, the neurologic deficit was mild in 17 patients, moderate in 21 patients, and severe in nine patients. Six patients had suffered from a TIA. The mean interval between stroke and MR imaging was 6 days (± 4 days).
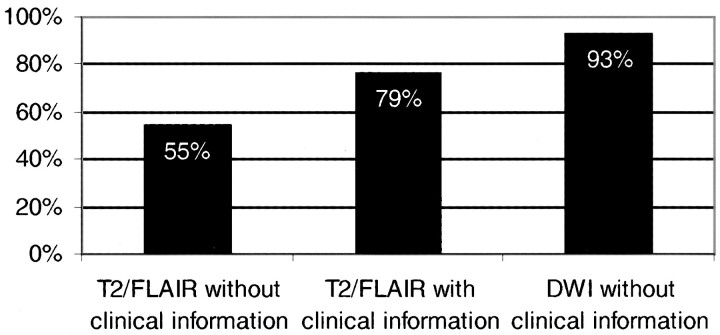
DWI unequivocally delineated recent ischemic lesions in 39 patients (74%). T2-weighted images were typical of subacute infarction in 23 patients (43%), and, when supplemented by clinical information, they enabled identification of the area of recent ischemic damage in another 10 patients (19%). Seven patients had false-negative findings on T2-weighted images (ie, subacute lesions were missed as compared with DWI), caused by 1) absence of signal abnormalities (in two patients imaged 26 and 36 hours, respectively, after stroke); 2) damage at sites unrelated to symptoms (in three patients, two with a TIA); and 3) lesion diameter of only 2 to 3 mm (in two patients). As compared with T2-weighted imaging, DWI missed recent ischemic damage (ie, was falsely negative) in only one patient, who was examined 8 days after stroke and had small subcortical lesions. Figure 1 compares the probability of lesion detection between DWI and T2-weighted imaging with and without clinical information for the subgroup of 41 patients with evidence of subacute ischemic damage on any of the MR sequences used, including contrast-enhanced T1-weighted images.
fig 1.
Graph compares the probability of lesion detection for the subgroup of 41 patients with evidence of subacute ischemic damage on any of the MR sequences used, including contrast-enhanced T1-weighted images. The first and second columns indicate the percentage of patients with a subacute lesion on T2-weighted sequences without and with knowledge of the patients' symptoms. The third column shows the relative sensitivity of DWI without clinical information
DWI showed multiple areas of recent ischemic damage in 10 patients. In three of those patients, lesions were distributed over different vascular territories from those expected on the basis of the conventional MR series. On T2-weighted images, dissemination of subacute lesions was seen in five patients, including the patient with a false-negative DWI study and the two patients with fewer lesions than seen on DWI sequences. Overall, DWI provided information not accessible with T2-weighted imaging in 15 patients (28%) either by documenting the recent ischemic lesion (seven patients) or by showing lesion multiplicity (eight patients). Examples are shown in Figures 2 through 4. Differences in the probability of a diagnostic contribution by DWI in relation to a patient's clinical features and concomitant imaging findings are provided in the Table.
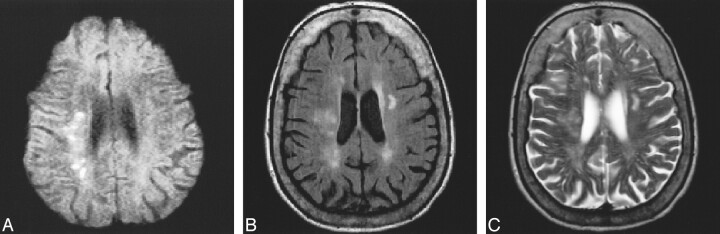
fig 2.
80-year-old patient with left hemiparesis and ataxia.
A–C, DWI (TR/TEdiff/TE = approximately 1500/115/18; 2 RR intervals) study (A) clearly shows multiple recent small ischemic lesions in the right centrum semiovale. These lesions are less visible on FLAIR image (6000/130/2, TI = 1900) (B) and T2-weighted-FSE image (2900/120/1) (C) and could not be identified as areas of subacute infarction on these sequences.
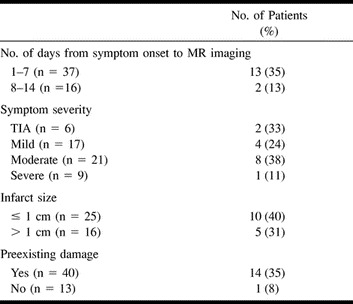
Clinical and imaging findings in patients undergoing diffusion-weighted MR imaging
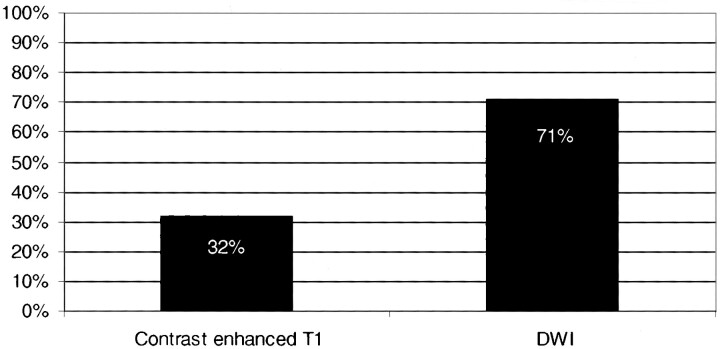
In the subgroup of 28 patients who received contrast material, DWI findings were positive for subacute ischemic lesions in 20 (Fig 5). Seven of these patients with diffusion-positive studies (ie, with evidence of subacute ischemic damage) had corresponding enhancement of lesions, whereas contrast-enhanced images were noncontributory in the other 13 (Fig 6). In two patients with diffusion-negative studies, however, focal enhancement of multiple small cortical lesions was noted. In these patients, the interval between stroke and MR imaging was 7 and 8 days, respectively. T2-weighted images showed corresponding signal abnormalities in one patient and was negative in the other. Multiplicity of lesions was evident in six patients on DWI studies (21%) but in only three patients on contrast-enhanced images (11%).
fig 5.
Graph compares the percentage of patients with subacute ischemic lesions on DWI and contrast-enhanced T1-weighted sequences in the subgroup of 28 patients in whom contrast-enhanced studies were obtained
fig 6.
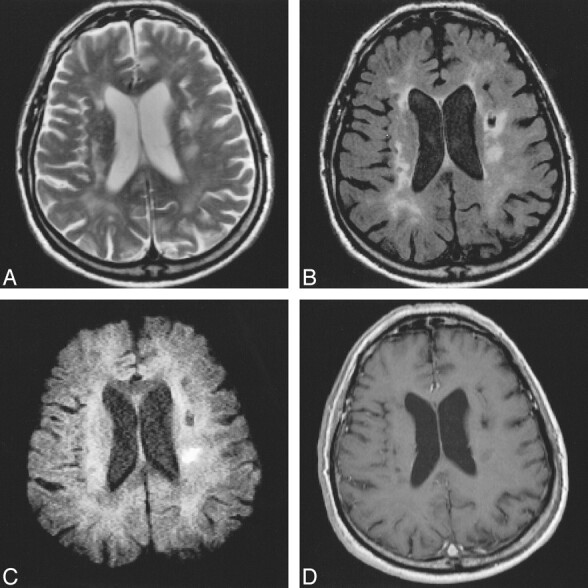
63-year-old patient with preexisting ischemic lesions.
A–D, Mild hemiparesis is well seen on FSE (2900/120/1) (A) and FLAIR (6000/130/2, TI = 1900) (B) sequences. DWI (approximately 1500/115/18; 2 RR intervals) sequence (C) depicts the area of recent ischemic damage 7 days after stroke. Contrast-enhanced MR image (588/14/2) (D) remains negative.
Discussion
Several studies have shown the capability of DWI to depict morphologic brain changes within the first hours of focal ischemia, and the usefulness of this technique for determining location and size of brain damage in the setting of acute stroke has been extensively documented (15–19). Our results provide clear evidence of a significant diagnostic contribution of DWI beyond this acute phase. In comparison with T2-weighted sequences, DWI proved to be superior in delineating areas of recent ischemic damage in about one third of all patients. We also found DWI to be more sensitive than contrast-enhanced MR imaging. This higher sensitivity of DWI served not only to pinpoint stroke-related infarcts but also helped to detect clinically unsuspected lesions in more than 10% of all patients.
The first few hours of stroke are certainly the most critical for attempts to salvage brain tissue. Therefore, morphologic information pertinent to early treatment decisions, such as the exact size of the infarction, can be crucial. Even thereafter, however, important clinical decisions may be facilitated by more detailed knowledge of the pattern of ischemic damage. Unequivocal imaging evidence of an ischemic lesion may serve both to confirm clinical suspicion of an ischemic event and to rule out other differential diagnoses. The pattern of damage per se may be indicative of stroke origin (eg, a multiplicity of acute lesions in different vascular territories would favor an embolic origin). Finally, counseling of the patient and prognostic assumptions regarding recovery are facilitated by clear insight into the extent and location of morphologic damage.
In general, ischemic lesions are well seen on conventional T2-weighted MR images, on which they appear as areas of high signal intensity. Characteristic lesion shape, confinement to specific vascular territories, and signs of mass effect are features indicative of an acute lesion. However, these characteristics are not always present, especially not in the case of small lesions. The coexistence of previous focal and diffuse ischemic brain damage with a similar signal intensity to that of acute lesions on conventional T2-weighted images may further complicate the delineation of recent ischemic damage. In contrast, delineation of acute infarcts by DWI is based on the specific changes in the motion of water molecules that follow ischemic damage (ie, restricted diffusion relative to normal brain). Ischemic lesions appear bright on DWI sequences only for a few days, and are easily separable from normal brain tissue or previous ischemic brain damage, including incidental white matter hyperintensities (13, 20). This is in complete accordance with our findings. The contribution of DWI was greatest for the detection of small lesions within the first few days after stroke, and in patients with preexisting brain damage. At around 1 week, the apparent diffusion coefficient (ADC) within ischemic brain parenchyma starts to rise and goes through a period of pseudonormalization before values become higher than those of normal tissue in areas that have undergone necrosis (13). Inversely, the sensitivity of DWI for detecting recent ischemic damage starts to diminish, and may even fail to pinpoint such lesions, especially around 1 week after stroke, as seen in some of our patients. In the subacute period, contrast arises from both T2 shine-through and decreased ADC. At a certain point after ADC pseudonormalization, the contribution to MR signal contrast coming from prolonged T2 values is compensated by the contrast contribution coming from increased ADC on DWI sequences.
The superiority of DWI over T2-weighted MR imaging observed in the present study is not unexpected on the basis of theoretical considerations and has also been mentioned in previous reports on the use of DWI in stroke patients (1–5, 17–19, 21). It appears of interest, however, that the contribution of DWI was not limited to depicting the clinically apparent infarct and superseded that of contrast-enhanced MR imaging. In seven patients, DWI also identified clinically silent ischemic lesions as recent in origin. This finding has important pathophysiological implications. Delineation of acute lesions in various vascular territories is almost pathognomonic for a cardiogenic or aortic source of embolism. Multiple lesions within one vascular territory, on the other hand, suggest artery-to-artery embolization or damage from debris of a larger embolus that has undergone partial lysis. Such a mechanism has been suggested to explain the finding of small ischemic lesions distant from clinically eloquent areas and could now be documented by DWI. In this context, DWI also helped to prove the timely relation of ischemic lesions in two of our patients with a TIA, which supports earlier observations of associated ischemic lesions on MR images in more than 20% of TIA patients (22).
Overall, the rate of patients with ischemic infarcts was lower on both conventional sequences and DWI as compared with that in previous series of stroke patients (16–19). The use of a moderate b value and the fact that we performed DWI in the axial plane might have contributed. Another explanation could be pseudonormalization of the DWI abnormality in patients scanned more than a week after the ischemic event. The study population itself, however, appears to offer an even better explanation for the discrepancy in observed sensitivities between our study and most of the recent literature. In an attempt to reflect common clinical practice with ready access to MR imaging, we did not select for patients with a clear-cut stroke only but also included those with ambiguous clinical findings. Moreover, there was certainly some bias toward patients in whom CT had not rendered a satisfactory clinicoradiologic correlation. Both these facts are likely to have favored the investigation of patients with transient or minute ischemic lesions. The fact that the sensitivity of both conventional sequences and of DWI were lower in our series than in previous reports supports this assumption. Therefore, the absence of multiaxial DWI or the use of a moderate b value appear to have been of subordinate importance.
An essentially sufficient sensitivity of the DWI technique used is also supported by the detection of multiple, clinically unexpected, subacute lesions in 10 of our patients. Certainly, the possibility of having missed some lesions by pseudonormalization of a DWI abnormality cannot be excluded. Otherwise, the large proportion of patients with preexisting brain damage will have tended to highlight the contribution of DWI in our study.
Diffusion anisotropy of the brain parenchyma did not cause difficulties in interpreting the DWI studies in our patients. This problem may have been mitigated by the moderate diffusion weighting used in our series. Although this is at the cost of the contrast-to-noise ratio relative to DWI, which has a higher b factor, it carries the advantage of less pronounced diffusion anisotropy. Except in two patients, who were examined only 26 hours and 36 hours, respectively, after stroke, recent DWI lesions always showed corresponding signal changes on T2-weighted images, which argues against a false-positive interpretation. Because of the clear visual delineation of subacute ischemic lesions, we also did not perform measurements of the diffusion coefficient. Some investigators consider ADC calculation and trace-weighted DWI necessary for obtaining precise results (23), although others agree on the high accuracy of single-direction DWI along one of the principal axes, which is also desirable for the sake of clinical practicability. The high accuracy of only orthogonal-axis DWI has also been documented in a more recent comparison of DWI data processing methods (13, 15, 24, 25).
Conclusion
DWI is known to excel in displaying acute cerebral ischemic damage. We now have also demonstrated the clear advantages of DWI over conventional T2-weighted sequences and contrast-enhanced imaging during the subacute period of stroke. The overall higher sensitivity of DWI for lesion detection, especially in patients with preexisting cerebral ischemic damage or in those with small lesions, may help to confirm the diagnosis of ischemic stroke and to clarify the origin of clinical symptoms. In parallel, evidence of lesion multiplicity can add information on stroke origin. These contributions to patient management clearly argue for an inclusion of DWI in standard MR protocols for the evaluation of stroke patients.
fig 3.
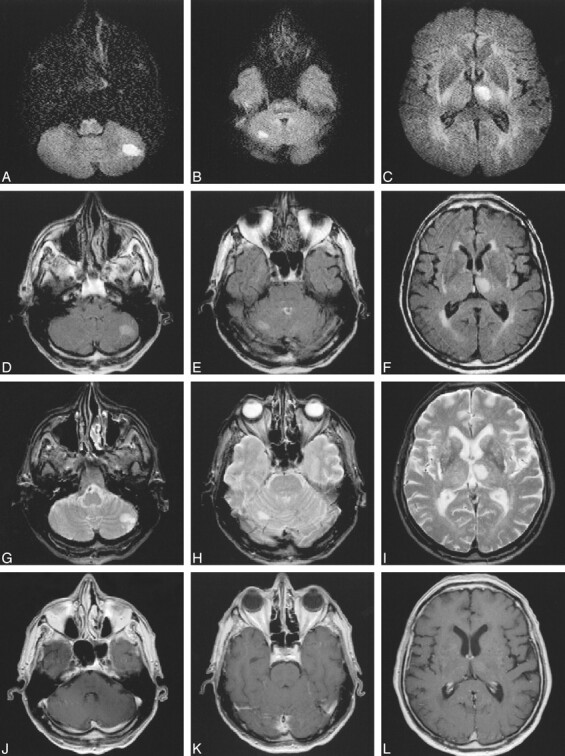
63-year-old patient with right hemiparesis.
A–L, Three days after stroke, DWI studies (approximately 1500/115/18; 2 RR intervals) (A–C) show two more clinically unexpected ischemic lesions in the cerebellum (A, B), suggestive of embolism. These two lesions would not have been definitively labeled as subacute on FLAIR (D–F) (6000/130/2, TI = 1900) or T2-weighted (2500/90/1) (G–I) sequences, especially in the presence of negative findings on contrast-enhanced images (556/14/2) (J–L).
fig 4.
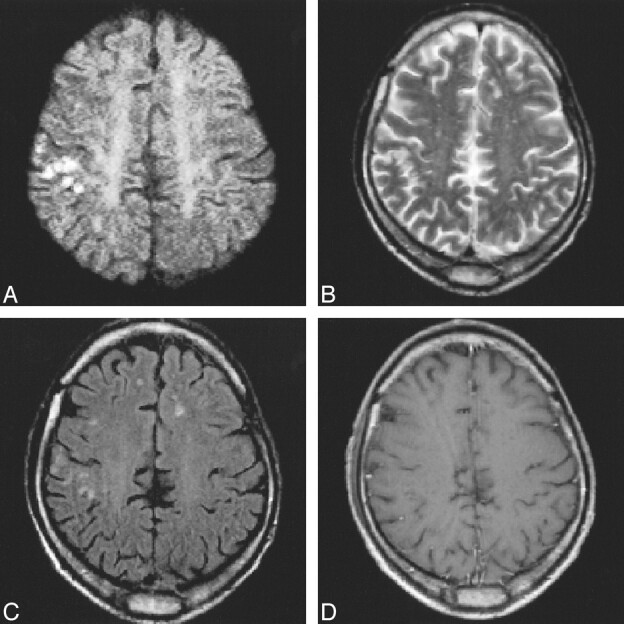
A–D, Multiple tiny cortico-subcortical lesions in the right motor region on DWI (approximately 1500/115/18; 2 RR intervals) study (A) confirm the ischemic origin of mild hemiparesis 4 days after stroke. These changes might have gone undetected on T2-weighted FSE (2900/120/1) (B), FLAIR (6000/130/2, TI = 1900) (C), or contrast-enhanced (588/14/2) (D) images
Footnotes
Roland Bammer is supported by the Gemeinnützige Hertie Stiftung.
Address reprint requests to Franz Fazekas, MD, Department of Neurology, Karl-Franzens University, Auenbruggerplatz 22, A-8036 Graz, Austria.
References
- 1.Moseley ME, Kucharczyk J, Mintorovitch J, et al. Diffusion-weighted MR imaging of acute stroke: correlation with T2-weighted and magnetic susceptibility-enhanced MR imaging in cats. AJNR Am J Neuroradiol 1990;11:423-429 [PMC free article] [PubMed] [Google Scholar]
- 2.Beneviste H, Hedlund L, Johnson G. Mechanism of detection of acute cerebral ischemia in rats by diffusion-weighted magnetic resonance microscopy. Stroke 1992;23:746-754 [DOI] [PubMed] [Google Scholar]
- 3.Le Bihan D, Turner R, Douek P, Patronas N. Diffusion MR imaging: clinical applications. AJR Am J Roentgenol 1992;159:591-599 [DOI] [PubMed] [Google Scholar]
- 4.Chien D, Kwong KK, Gress DR, Buonanno FS, Buxton RB, Rosen BR. MR diffusion imaging of cerebral infarction in humans. AJNR Am J Neuroradiol 1992;13:1097-1102 [PMC free article] [PubMed] [Google Scholar]
- 5.Warach S, Chien D, Li W, Rontal MM, Edelman RR. Fast magnetic resonance diffusion-weighted imaging of acute human stroke. Neurology 1992;42:1717-1723 [DOI] [PubMed] [Google Scholar]
- 6.Warach S, Gaa J, Siewert B, Wielopolski P, Edelmann RR. Acute human stroke studies by whole brain echo planar diffusion-weighted magnetic resonance imaging. Ann Neurol 1995;37:231-241 [DOI] [PubMed] [Google Scholar]
- 7.Warach S, Dashe JF, Edelman RR. Clinical outcome in ischemic stroke predicted by early diffusion-weighted and perfusion magnetic resonance imaging: a preliminary analysis. J Cereb Blood Flow Metab 1996;16:53-59 [DOI] [PubMed] [Google Scholar]
- 8.Maeda M, Maley JE, Crosby DL, et al. Applications of contrast agents in the evaluation of stroke: conventional MR and echo-planar MR imaging. J Magn Reson Imaging 1997;7:23-28 [DOI] [PubMed] [Google Scholar]
- 9.Crain MR, Yuh WTC, Greene GM, et al. Cerebral ischemia: evaluation with contrast-enhanced MR imaging. AJNR Am J Neuroradiol 1991;12:631-639 [PMC free article] [PubMed] [Google Scholar]
- 10.Alster AD, Moody DM. Early cerebral infarction: gadopentetate dimeglumine enhancement. Radiology 1990;177:627-632 [DOI] [PubMed] [Google Scholar]
- 11.Miyashita K, Naritomi H, Sawada T, et al. Identification of recent lacunar lesions in cases of multiple small infarctions by magnetic resonance imaging. Stroke 1988;19:834-839 [DOI] [PubMed] [Google Scholar]
- 12.Mueller DP, Yuh TC, Fisher DJ, Chandran KB, Crain MR, Kim YH. Arterial enhancement in acute cerebral ischemia: clinical and angiographic correlation. AJNR Am J Neuroradiol 1993;14:661-668 [PMC free article] [PubMed] [Google Scholar]
- 13.Bammer R, Stollberger R, Augustin M, et al. Diffusion imaging using navigated interleaved echo planar imaging and a conventional gradient system. Radiology 1999;211:799-806 [DOI] [PubMed] [Google Scholar]
- 14.Adams RJ, Meador KJ, Sethi KD, Grotta JC, Thomson DS. Graded neurological scale for use in acute hemispheric stroke treatment protocols. Stroke 1987;18:665-669 [DOI] [PubMed] [Google Scholar]
- 15.Moseley ME, Cohen Y, Mintorovich J, et al. Early detection of regional cerebral ischemia in cats: comparison of diffusion- and T2-weighted MRI and spectroscopy. Magn Reson Med 1990;14:330-346 [DOI] [PubMed] [Google Scholar]
- 16.Minematsu k, Li L, Fisher M, Sotak CH, Davis MA, Fiandaca MS. Diffusion-weighted magnetic resonance imaging: rapid and quantitative detection of focal brain ischemia. Neurology 1992;42:235-240 [DOI] [PubMed] [Google Scholar]
- 17.Sorensen AG, Buonano FS, Gonzalez RG, et al. Hyperacute stroke: evaluation with combined multisection diffusion-weighted and hemodynamically weighted MR imaging. Radiology 1996;199:391-401 [DOI] [PubMed] [Google Scholar]
- 18.Lövblad KO, Laubach HJ, Baird AE, et al. Clinical experience with diffusion-weighted MR in patients with acute stroke. AJNR Am J Neuroradiol 1998;19:1061-1066 [PMC free article] [PubMed] [Google Scholar]
- 19.Singer M, Chong J, Lu D, Schonewille W, Tuhrim S, Atlas S. Diffusion weighted MRI in acute subcortical infarction. Stroke 1998;29:133-136 [DOI] [PubMed] [Google Scholar]
- 20.Schlaug G, Siewert B, Edelman RR, Warach S. Time course of the apparent diffusion coefficient (ADC) abnormality in human stroke. Neurology 1997;49:113-119 [DOI] [PubMed] [Google Scholar]
- 21.Bahn MM, Oser AB, Cross DT III. CT and MRI of stroke. J Magn Reson Imaging 1996;6:833-845 [DOI] [PubMed] [Google Scholar]
- 22.Fazekas F, Fazekas G, Schmidt R, Kapeller P, Offenbacher H. Magnetic resonance imaging correlates of transient cerebral ischemic attacks. Stroke 1996;27:607-611 [DOI] [PubMed] [Google Scholar]
- 23.Chong J, Lu D, Aragao F, et al. Diffusion-weighted MR of acute cerebral infarction: comparison of data processing methods. AJNR Am J Neuroradiol 1998;19:1733-1739 [PMC free article] [PubMed] [Google Scholar]
- 24.de Crespigny AJ, Marks MP, Enzmann DR, Moseley ME. Navigated diffusion imaging of normal and ischemic human brain. Magn Reson Med 1995;33:720-728 [DOI] [PubMed] [Google Scholar]
- 25.Ulug AM, Beauchamps N, Bryan RN. Absolute quantitation of diffusion constants in human stroke. Stroke 1997;28:483-490 [DOI] [PubMed] [Google Scholar]