Abstract
BACKGROUND AND PURPOSE: Carotid endarterectomy (CEA) is one of the most frequently performed operations in the United States. To offer patients a less invasive means to achieve the same goal, carotid artery stenting (CAS) is investigated as an alternative treatment to CEA.
METHODS: Three hundred ninety patients underwent CAS, with 451 vessels treated. CAS was performed using a coaxial system with a 7F 90-cm sheath for predilation, stent placement, and stent dilation. Pretreatment antiplatelet therapy was administered. We currently practice same-day admissions and 23-hour discharges.
RESULTS: The technical success rate was 98%. The 30-day mortality/morbidity rates were as follows: death, 1.7% (two [0.5%] neurologic and five [1.2%] systemic] major strokes, 0.9% (two of four were related to the intervention); minor strokes, 5.5%. Among 25 patients who suffered minor strokes, 14 achieved complete recovery. On an annual basis, the incidence of minor stroke declined from 6.8% (1994–1995), to 5.8% (1995–1996), 5.3% (1996–1997), and then 4% (1997–1998), with no major strokes or neurologic deaths occurring during the 1997 to 1998 period.
CONCLUSION: CAS is an effective treatment for carotid stenosis. With proper selection of patients and meticulous technique, complication rates compare favorably with those of CEA.
Carotid artery stenting (CAS) is currently being investigated as an alternative treatment to carotid endarterectomy (CEA) (1–6). The goal of both procedures is the prevention of stroke from extracranial carotid artery occlusive disease. Carotid stenting, compared with surgery, offers patients a less invasive and traumatic means of achieving this goal. The efficacy of carotid stenting in preventing stroke depends on the ability of the operator to achieve complication-free results. The purpose of this article is to describe the technical details and patient selection factors that have allowed us to improve the outcomes from this procedure.
Methods
Patients and Techniques
Between September 1994 and September 1998, we performed CAS on 390 patients (451 carotid arteries; 421 total procedures). Sixty-one patients (16%) underwent treatment for bilateral disease. This was performed simultaneously with the CAS in 30 patients and was staged in 31. To be considered for stenting, symptomatic patients required at least 50% stenosis (7–9) and asymptomatic patients required at least 70% diameter narrowing (10) by angiographic North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria (7). All patients were treated under a prospectively defined Institutional Review Board-approved protocol, and all gave written informed consent for the procedure.
Patients Characteristics
The patients' ages ranged from 35 to 89 years (68 ± 10 years); 70% were male patients. Fifty-six percent of the stenotic arteries were symptomatic: 30% presented with transient ischemic attack, 25% with relatively recent stroke, and 1% with amaurosis fugax. Seventy percent were hypertensive, 62% had hypercholesterolemia, 31% were diabetic, and 70% had coronary artery disease. These conditions and a variety of other cardiac and noncardiac comorbid conditions combined with unfavorable surgical anatomy (retromandibular stenosis) would have made 89% ineligible for inclusion in the NASCET study (7). Seventy (17%) of the arteries treated had undergone previous CEA and showed significant restenosis, and 18 (4%) had undergone previous ipsilateral radiation for head or neck cancer. Forty-three (11%) of the patients had occlusion of the contralateral carotid artery.
Clinical Protocol
Patients are admitted the day of the procedure, and consent is obtained for brachiocephalic angiography and possible stent placement, should the lesion prove to be of significant severity and is anatomically suitable. All patients are referred for neurologic examination to document the preprocedural clinical neurologic status. All patients receive a National Institutes of Health Stroke Scale (NIHSS) score determined by a neurologist. Symptomatic patients or patients with history of stroke or transient ischemic attacks and those with abnormal results of the neurologic examination undergo CT or MR imaging (if this has not recently been performed) to document preprocedural pathologic changes. Before the stenting, all patients undergo brachiocephalic angiography (if this has not recently been performed). Patients are informed that CAS is considered an investigational procedure and that despite favorable medium-term (2 years) outcomes (11), long-term data are not yet available. Antiplatelet therapy is administered 2 to 4 days before the procedure: aspirin (325 mg administered daily), ticlopidin (Ticlid, 250 mg administered twice a day), and presently clopidogrel (Plavix, 75 mg administered twice a day), are administered for 2 to 4 days before the procedure. In all cases, patients should have received ticlopidin (total dose, 500 mg) or clopidogrel (total dose, 300 mg) before the intervention. Data were collected prospectively, and a special case report form was filled out by a dedicated research nurse. Neurologic outcome was based on pre- and postprocedural (24 hours) examinations by a neurologist. Patients who developed periprocedural events underwent formal neurologic examinations and received NIHSS scores at 30 days. Follow-up on all other patients was obtained by telephonic contact with patients and referring physicians.
Procedural Considerations
Angiography and stenting are performed with the patient under local anesthesia. The neurologic status of the patient is monitored after each step of the procedure (12). Throughout the intervention, continuous electrocardiography with monitoring of the heart rate and blood pressure is performed.
Definitions
Technical success was defined as the ability to access the carotid artery and successfully stent the lesion with residual stenosis of no more than 20%.
Study end points were defined as an occurrence within 30 days of minor stroke, major stroke, death, or myocardial infarction.
Minor stroke was defined as an increase in the NIHSS score of less than 3, with complete resolution or no significant disability at 30 days.
Major stroke was defined as an increase in the NIHSS score of 3 or more, with significant disability at 30 days.
Current Technique
Our current technique can best be discussed in terms of initial angiography, access sheath placement, predilation, and stenting.
Initial Angiography
In the vast majority of elderly patients, only one 5F catheter (VTK Thorocon NB, 100 cm; Cook, Inc., Bloomington, IN) is needed to catheterize all brachiocephalic arteries. With exceptions, in extremely dilated aortic arches, a sidewinder, curved catheter is used (Simmons 3 curve). The diagnostic angiography consists of visualization of the origins of the brachiocephalic arteries from the aortic arch (by selective injections), both carotid bifurcations in several projections, both vertebral arteries, and intracranial study of both carotid arteries and the dominant vertebral artery.
Access Sheath Placement
Once the diagnostic study is completed and the stenotic internal carotid artery is identified, the 5F catheter is advanced, using the 0.038-inch glide wire, into the ipsilateral external carotid artery. The glide wire is then withdrawn and replaced with an extra stiff 0.038-inch exchange wire (0.038-inch Extra Stiff Amplatz Wire, 260 cm; Cook, Inc.). The 5F catheter is withdrawn, and the 7F 90-cm guiding sheath (Shuttle; Cook, Inc.) is then advanced into the common carotid artery (Figs 1A and 2D) over the exchange Amplatz wire, which is anchored in the external carotid artery. If the brachiocephalic diagnostic angiography has previously been performed and the target lesion identified, the stenting procedure begins by placing the 7F 90-cm Shuttle sheath, via the femoral approach, into the upper thoracic aorta. After withdrawing the inner dilator from the sheaths, the 125-cm 5F catheter (VTK Thorocon NB, 125 cm) is introduced into the 7F sheath. Care must be taken not to advance the 7F sheath too closely to the aortic arch because this will decrease the maneuverability of the 5F catheter. The common carotid artery is then catheterized with the 125-cm 5F catheter, and this catheter is advanced into the external carotid artery (road mapping is very useful). Using the appropriate guidewire (0.038-inch glide wire or extra stiff 0.038-inch Amplatz wire), depending on the arch extension and carotid artery tortuosity, the 7F sheath is advanced over the 5F catheter into the common carotid artery, just proximally to the stenosis. If the advancement of the sheath over the 5F catheter is not smooth, the 5F catheter is removed, replaced with the sheath's inner introducer, and then the sheath is advanced into the common carotid artery. There are modifications of this technique, especially if the stenosis is located in mid or distal segments of the common carotid artery or if the external carotid artery cannot be catheterized. The 5F catheter guidewire assembly, over which the 7F sheath is placed in the common carotid artery, is kept below the stenosis or bifurcation (Amplatz exchange J-wire is used). Exceptionally, in cases of significant aortic arch elongation or common carotid artery tortuosity, the stenosis is traversed with a glide wire and 4F or 5F catheter. Next, using an Amplatz wire, the 7F sheath is advanced proximally to the stenosis. This technique is also used in ostial and very proximal carotid stenoses. One should be aware that by placing the 7F sheath in the common carotid artery, especially if the carotid artery is tortuous (Fig 2A), the bifurcation is displaced upward and kinks can be created on the internal carotid artery (Fig 2D and E). These disappear once the sheath is withdrawn but can complicate the stenting procedure. As soon as the 7F sheath is placed into the arterial system, 4.000 to 5.000 units of heparin are administered through the sheath to raise the activated clotting time to no more than 250 s (Hemotec Company method).
fig 1.
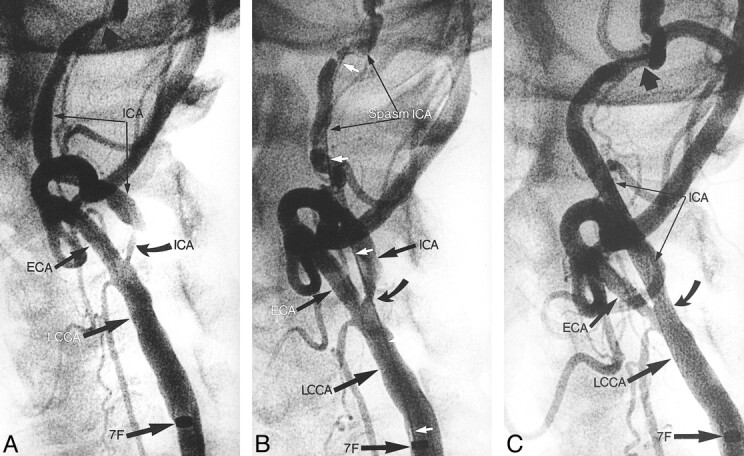
Angiograms from the case of an 80-year-old male patient with bilateral internal carotid artery stenosis.
A, Angiogram shows 80% stenoses in the left internal carotid artery (ICA) (curved arrow). A 7F sheath (7F) was inserted into the left common carotid artery (LCCA). Distal, independent stenosis on the internal carotid artery (thick black arrow) can be seen. ECA, external carotid artery.
B, Angiogram shows status after predilation (curved arrow) of the internal carotid artery with a 4-mm balloon over a 0.018-inch guidewire (white arrows). Spasm on the distal internal carotid artery can be seen.
C, Angiogram obtained after the CAS control study. A 10 × 20 Wallstent was used, dilated with a 5 × 20 Symmetry balloon. Minimal residual spasm can be seen. Independent distal stenosis persists (wide black arrow).
fig 2.
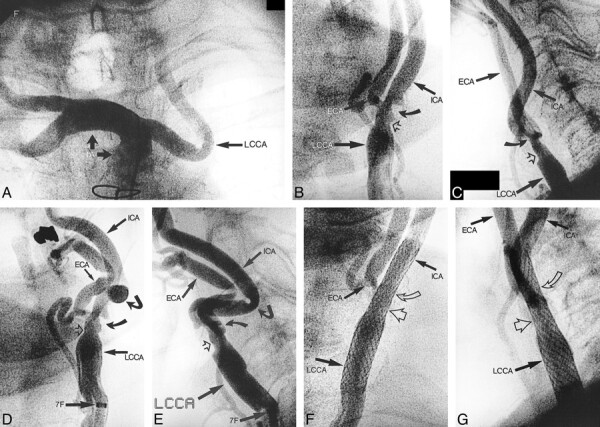
Angiograms from the case of an 87-year-old female patient with occlusion of the right internal carotid artery and 65% symptomatic, ulcerated stenosis on the left common and internal carotid arteries.
A, Angiogram of the innominate artery (IA) shows a 90-degree take-off of the left common carotid artery (LCCA), with more distal tortuosity.
B, Anteroposterior projection angiogram of the left common carotid artery (LCCA) (5F catheter in the ostium). Sixty-five percent ulcerated stenosis (curved arrow) on the internal carotid artery (ICA) and on the common carotid artery (open arrow) can be seen. ECA, external carotid artery.
C, Lateral projection.
D, Anteroposterior projection angiogram of the left common carotid artery (LCCA), obtained through a 7F sheath placed in the distal left common carotid artery. By upward displacement of the bifurcation, kink (angled arrow) developed in the proximal internal carotid artery. 7F, distal tip of the 7F sheath; ECA, external carotid artery; open arrow, stenosis in the distal left common carotid artery; curved arrow, ulcerated stenosis in the internal carotid artery.
E, Lateral projection.
F, Anteroposterior projection angiogram of the left common carotid artery (LCCA), obtained after CAS was performed. A 10 × 20 Wallstent was used, dilated with a 5.5 × 20 Symmetry balloon. The 7F sheath is removed; the 5F catheter is in the ostium of the left common carotid artery. Open curved arrow, previous location of the ulcerated stenosis on the internal carotid artery.
G, Lateral projection.
Predilation
Arteriography through the sheath, in appropriate angulation, is performed to show the maximum tightness of the stenosis. Quantitative carotid arteriography is performed in this projection to measure the percent diameter of the stenosis and also the diameter of the common carotid artery. Then the optimal angulation is found to perform the intervention. This projection is not necessarily the one to show the maximum stenosis severity, but the one separating the internal and external carotid arteries and to show bony landmarks comfortably. To monitor neurologic status continuously, particularly the strength of the contralateral upper extremity, a squeeze toy is placed in patient's hand (12). The stenotic lesion is crossed with a steerable 0.014-inch guidewire. The wire is guided with small contrast injections through the sheath rather than with road mapping, which is subject to misregistration (especially in tight stenosis) because of the patient's breathing. Selection of the wire depends on the tightness, location, lengths, angulation, and eccentricity of the stenosis and on the anatomy of the bifurcation (Figs 1A and 2D and E). The tip of the 0.014-inch wire is shaped appropriately. A variety of 0.014-inch wires are available. We commonly use exchange-length 0.014-inch Balance Wire (Guidant, Inc., Temecula, CA) or 0.014-inch Choice PT Wire (SciMed Inc., Maple Grove, MN). After crossing the stenosis, the tip of the wire is placed close to the skull base. If the internal carotid artery is kinked or presents coils or tortuosities, the wire is passed through, distally, to the level of the skull base. For predilation of the stenosis, we routinely use 0.018-inch compatible balloons, such as the Cobra 4 × 40-mm coronary balloon (SciMed Inc.) and, more recently, the Savvy 4 × 40 mm balloon (Cordis Corp., Miami, FL). The pressure used for predilation is nominal for the balloon used. We use higher pressure (14–16 atm) only in cases of heavily calcified stenoses. The duration of the predilation depends on the appearance and behavior of the balloon. If the balloon immediately attains its full shape, the predilation time is short (10–20 s). If the balloon attains its full shape slowly, the predilation time is prolonged (≤120 s), again, especially in calcified lesions, which have a tendency for recoiling. We routinely inflate the balloon only once, and we vary the inflation time. If the stenosis is preocclusive, we use a steplike predilation method. First, we predilate the stenosis with a 2 × 40-mm balloon (Ranger, SciMed Inc.). After this predilation, a second predilation, using the 4-mm balloon, is performed. After the predilation, if needed, the 0.018-inch compatible balloon catheter is advanced distally into the internal carotid artery and the 0.014-inch wire is changed for the 0.018-inch exchange wire (Roadrunner; Cook, Inc.). Again, the tip of the 0.018-inch wire should be located close to the skull base and must be passed through all kinks and tortuosities of the internal carotid artery (Fig 1B). Occasionally, if the stenosis is not significantly irregular or ulcerated and if it is less than 80% in diameter and the internal carotid artery is not tortuous, we traverse the stenosis directly with the 0.018-inch Roadrunner wire. Very rarely, usually in cases of heavily calcified lesions, if after predilation with a 4-mm balloon the stent does not easily pass through the stenosis, a 5-mm balloon is used for additional predilation. After the predilation and placement of the 0.018-inch guidewire, control arteriography is performed.
Stent Deployment and Postdilation
Presently, we are using only self-expanding stents. In our early experience, we worked with balloon-expandable stents (3, 6, 13). This practice was abandoned with three exceptions: 1) when the ostium of the common carotid artery is treated and the proximal end of the stent has to be placed with precision; 2) when the most distal neck segment (prepetrous segment) of the internal carotid artery is treated (present delivery systems for the self-expanding stent can cause dissections in the petrous portion of the internal carotid artery, ie, in any sharp bends of the internal carotid artery); and 3) when the self-expandable stent cannot be smoothly advanced through the stenosis after predilation with a 5-mm balloon (usually in heavily calcified stenoses). The self-expanding stent is deployed using the vertebral bodies as landmarks. We oversize the stents, using 8- or 10-mm-wide stents when the proximal end is placed in the common carotid artery and 7- or 8-mm-wide stents when placed exclusively in the internal carotid artery. The self-expandable stent is postdilated with a 5, 5.5, or 6 × 20-mm balloon (Symmetry; MediTech, Watertown, MA; and Savvy, Cordis Corp.) over the 0.018-inch wire, depending on the size of the internal carotid artery. Nominal pressure is used to expand the balloon and the stent fully. The balloon is deflated slowly. High pressures are no longer used. Only in heavily calcified stenosis do we postdilate with Titan balloons (Cordis Corp.), which accept higher pressures. In the majority of the cases, the stent is placed across the bifurcation into the common carotid artery, crossing the origin of the external carotid artery (Figs 1C and 2F and G). To cover the external carotid artery with the stent does not cause problems; our follow-up arteriograms showed the external carotid artery to be patent, with rare exceptions (6). After the intervention, the long sheath is pulled over the wire into the iliac artery and replaced with a short sheath of appropriate size, which is removed when the activated clotting time is less than 150 s. Patients are discharged the next morning after undergoing neurologic examination. Patients continue to take soluble aspirin (325 mg) indefinitely and Plavix (75 mg twice a day) for 4 weeks. Presently, we use a femoral artery suture device (Perclose; Perclose Inc., Redwood City, CA), which enables us to discharge patients the same day (13).
Results
Procedural Data
Technical success was achieved in 98% of the cases. In seven of these procedures, catheter access into the carotid artery could not be obtained because of extreme tortuosity, and in two cases, the procedure was aborted before intervention after air emboli occurred during the sheath placement.
There was one stent thrombosis. The mean percent stenosis was reduced from 74 ± 15% to 5 ± 10%. Self-expanding Wallstents were used in 72% of the total arteries, and balloon-expandable stents were used in the remaining arteries. In eight cases, the external carotid artery was severely compromised and successfully treated by balloon dilation through the stent.
Complications
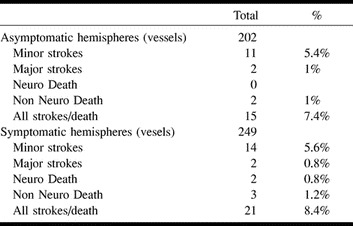
The 30-day events are detailed in the Table. The overall combined stroke and death rate at 30 days was 7.9%. One patient had non-Q wave myocardial infarction; no cranial nerve palsies were observed. Seven (1.7%) deaths occurred; two (0.5%) were neurologic and five (1.2%) were systemic. Neurologic death occurred in one patient after stroke because of carotid artery rupture (too large a postdilation balloon). The rupture was sealed, but major stroke developed and the patient died as a result of subsequent multiorgan failure. The second patient experienced rupture of a previously unknown internal carotid artery aneurysm after CAS was performed simultaneously on both carotid arteries, one of which was completely occluded (the side of the aneurysm). There was a total of four (0.9%) major strokes. One major stroke was related to the acute stent thrombosis, and one was related to embolus in the middle cerebral artery with failed neurovascular rescue. One major stroke was unrelated to the CAS and was due to the embolus in the opposite middle cerebral artery (3 days after procedure) from the prosthetic mitral valve in a patient with chronic atrial fibrilation, and one was due to a coronary rotoblader procedure that was complicated by perforation and hypovolemic shock. There were 25 (5.5%) minor nondisabling strokes. Of the patients who experienced minor strokes, 14 (3.1%) had an increase in the NIHSS score of 1 and completely recovered within 7 days, most within 24 to 48 hours. During the last 12-month period of this series, we experienced only four minor strokes, with no major strokes or mortalities.
Discussion
CAS is an alternative revascularization technique for extracranial carotid stenotic disease. In September 1994, a multidisciplinary group of physicians, comprised of interventional neuroradiologists and interventional cardiologists (one of whom was board-certified in neurology), started to investigate CAS. From the beginning, the group based its technique on the premise that combining angioplasty with elective intravascular stent placement will increase the reliability and safety of the method. The first series of patients in this study who underwent stenting was reported in 1995 (3).
Refinements in patient and lesion selection and interventional technique as well as an increase in our understanding of several factors that improve outcome (15) have resulted in reducing the incidence of neurologic complications. From an 8.2% complication rate in 1994 to 1995, we reduced the complication rate to 4% in 1997 to 1998. On an annual basis, the incidence of minor embolic stroke has declined from 6.8% (1994–1995), to 5.8% (1995–1996), 5.3% (1996–1997), and 4% (including one retinal artery embolus 2 weeks after the intervention) (1997–1998).
In the clinical protocol, we place great emphasis on antiplatelet therapy before and after CAS. In our judgment, the low rate of acute and delayed stent thrombosis and elimination of acute poststenting embolic events is predicated on correct and compulsive doses of adjunctive antiplatelet therapy.
The patient selection was modified based on analysis of complications related to different groups of patients. Advanced age was the most important predictor of procedural neurologic complications, especially in patients older than 80 years (15). Patients with several comorbidities, especially those associated with significant hypertension, and patients who have recently suffered stroke, are also more prone to neurologic complications (11, 15). In our opinion, patients with severe brain atrophy/dementia and patients with Alzheimer's disease do not tolerate carotid stenting well. We do not accept patients with severe renal impairment, which precludes the safe use of a contrast agent, and we do not accept patients who are unable to tolerate appropriate doses of antiplatelet agents. On the other hand, our analysis showed that a number of higher risk situations for CEA represents ideal indications for CAS, such as restenosis after CEA (16), stenosis due to previous neck radiation and radical neck surgery (17), and lesions in distal internal carotid artery or involving high, retromandibular bifurcation. Sex, the presence or absence of neurologic symptoms, coronary artery disease, diabetes mellitus, hypercholesterolemia, smoking, and the presence of bilateral carotid lesions, ulcerations, or contralateral carotid occlusion do not significantly influence the incidence of neurologic complications (15).
In general, it should be noted that more systemic factors and comorbidities increase the risks associated with CEA, whereas local anatomic and lesion factors increase the risks associated with CAS. At the beginning of our experience, the only contraindication for CAS was pedunculated thrombus at the lesion site (thrombi are more easily seen on 7.5 or 15 frame/s cine angiograms than on digital subtraction angiograms). By analyzing our complications, it became apparent that the lesion severity (≥90% diameter stenoses) and the length and multiplicity of the stenoses were associated with more embolic complications (15). In our experience, patients at higher risk of neurologic complications are those with severely tortuous, calcified, and atherosclerotic carotid vessels and carotid stenosis with severe concentric calcifications. Significant kink, tortuosity, and angulated take-off of the internal carotid artery brings significant technical difficulties to CAS and is also prone to more complications. We completely abandoned recanalizations of occluded internal carotid arteries, not because they were associated with more embolic complications but because of a high rate of postrecanalization intracerebral hemorrhages.
Despite the presence of “difficult” lesions and anatomic situations and the presence of thrombotic, friable, atherosclerotic plaque in many patients with carotid disease, meticulous technique can minimize the risk of cerebral emboli. The initial complete brachiocephalic angiography not only remains the most reliable method for assessment of the precise degree of carotid artery stenosis but also reveals all anatomic conditions that are unfavorable to CAS and that will indicate that surgical therapy should be the intervention of choice. Preprocedural knowledge of contralateral carotid stenosis occlusion and the conditions of intracranial circulation (isolated hemisphere, collateral supply) can subtly modify the CAS technique (shorter balloon inflations). Significant distal and intracranial internal carotid artery stenosis that slows the blood flow is unfavorable to CAS, because slow flow is prone to acute stent thrombosis.
The coaxial technique with guiding sheath placement ascertains continuous access to the common carotid artery. This is important when the access to the bifurcation is more difficult (elongated arch, tortuosity of the common carotid artery), when the passage of the guidewire through the stenosis is difficult (eccentric stenosis, ulcerations, internal carotid artery kinks and tortuosities, angulated take-off of the internal carotid artery), and when complication develops (dissection, intracranial embolism). The disadvantages of using a coaxial system (sheath or guiding catheter) are as follows: 1) there is wider access into the common femoral artery; 2) the placement of the sheath can prolong the time of the intervention; 3) the sheath can change the anatomy of the bifurcation; and 4) eventually, dissection can be created in the innominate or common carotid artery by careless advancement of the sheath. The access sheath we use is thin-walled and kink- and pressure-resistant, with good flexibility. The sheath has an open-ended Tuohy-Borst manual adjusting valve seal, which permits unimpeded catheter or guidewire introduction and ease in avoiding air or thrombus entrapment. The side-arm fitting allows intermittent or continuous flushing and contrast injection and also allows for continuous intraarterial blood pressure monitoring. The sheath can be also used as a basis for precise measurement of the internal and common carotid artery size (quantitative carotid angiography) (a sheath outer diameter of 3 mm = 9 F). Heparin is injected through the sheath. This prevents formation of the clots within the sheath. The activated clotting time is raised to no more than 250 s. A higher rate could increase the tendency toward brain hemorrhage.
The experimental work of Ohki et al (18) has shown that embolic debris is potentially released with “primary stenting” without predilation and during lesion dilation with large, peripheral-type balloons. Our experience from coronary angioplasty taught us to use same technique for CAS. We never cross the stenosis with a wire larger than 0.018 inch. The Nitinol tips of the 0.014- and 0.018-inch wires are conveniently radiopaque, soft, flexible, and nontraumatic to the internal carotid artery. Throughout all steps of CAS, we are very careful to keep the position of the tip of the wire close to the skull base; the wire must be advanced through all kinks and tortuosities in the internal carotid artery. The position of the wire provides us the assurance that if a dissection develops distally to the stent, it can be easily approached and repaired. For routine predilation, we use low-profile coronary 4 × 40-mm balloons, which accept 0.018-inch wires. Four-millimeter balloon predilation does not cause major occlusive dissections and is sufficient for passage of the stent (Fig 1B). The inflation pressure we use is nominal, and the deflation should be slow and full before withdrawing the balloon. The length of the balloon (40-mm) is advantageous in that it prevents the balloon from sliding out from the stenosis during inflation. As mentioned, the 0.014-inch wire, if used, is exchanged for a 0.018-inch wire before stent placement. First, it is easier to advance and accurately place the stent over the stiffer wire, and second, the more robust wire straightens the internal carotid artery, again facilitating the delivery and deployment of the stent (Fig 1B). We do not practice primary stenting without predilation; it is our impression that later postdilation of the constricted stent is associated with more “scissoring” of the stent wires on plaque and eventual subsequent embolization. After predilation control arteriography is performed, predilation and placement of the 0.018-inch wire can change the anatomic characteristics of the bifurcation in relationship to the osseous neck structures, which we use for stent placement.
For stenting we use self-expanding stents. A balloon-expandable stent has several disadvantages when deployed within the carotid bifurcation or its vicinity. Frequently, more than one stent is needed. The stent has to be differentially expanded to accommodate the size of the internal carotid artery, bifurcation, and common carotid artery; thus, more than one balloon is needed for appropriate dilation. The delivery balloon can rupture while deploying the stent; there may be difficulties in advancing the balloon-stent assembly (especially a 20-mm stent) through the guiding sheath if the aortic arch is significantly distended or the proximal common carotid artery is tortuous. The balloon-expandable stents tend to occlude the external carotid artery more frequently. They may also undergo deformation (13) with appropriate restenosis. Three exceptions when using the balloon-expandable stent have been noted (see “Stenting: Stent Deployment and Postdilation”). In heavily calcified stenosis, the postdilation recoil is common (even with 5-mm balloon predilation), and forcing the current, relatively high profile stent delivery systems through the stenosis may break off plaque and cause embolization. In this situation, a short Palmaz (P104) stent or AVE (5-mm) stent is placed to hold the stenosis open before passing a definitive self-expanding stent.
Self-expanding stents have several advantages; with exceptions, only one stent is needed. They are easily deployed, especially knowing that the distal end of the stent has to be deployed from within the healthy part of the internal carotid artery. It is not significantly important where the proximal end of the stent will be located in the common carotid artery. They can be easily deployed using vertebral bodies as landmarks and are easily postdilated. The unconstrained diameter of the self-expanding stent to be deployed should be at least 1 to 2 mm larger than the largest vessel segment to be covered by the stent (ie, usually the common carotid artery). In the past, we used 8- and 10-mm-diameter stents. Lately, we have used almost exclusively 10-mm-diameter stents (Figs 1C and 2F and G) because the stent mesh covering the diseased vessel is tighter, minimizing the possibility of emboli during postdilation. If the stent is placed only in the internal carotid artery, we use an 8-mm-diameter stent. Although the internal carotid artery is commonly 2 to 3 mm smaller than the common carotid artery, follow-up studies have shown that oversizing the stent in the internal carotid artery does not cause any acute or late problems (19). The length of the stent depends on the length of the stenosis being 20 to 40 mm. We try to avoid placing the distal end of the stent into kinks and tortuosities of the internal carotid artery. These cannot be eliminated and are only displaced distally and become more exaggerated. In the past, the only available self-expanding stents were Wallstents (Wallstent, 135-cm usable delivery length; Boston Scient. Vasc., Minneapolis, MN). More recently, we have gained experience with nonshortening Nitinol self-expanding stents (Memotherm, Angiomed Bard Inc.; Smart Stent, Cordis Corp.; Acculink, Guidant, Inc.). These stents are less rigid, do not straighten the internal carotid artery as much as the Wallstents do, and can be more precisely placed using the distal and proximal markers. An important technical point is to release 3 to 5 mm of the stent distally and wait for the stent to expand fully and stabilize against the vessel wall before releasing the remainder of the stent. All the Nitinol stents have the tendency to “jump” distally if released too fast. Some of the newer stents need only a 6F delivery system. In our experience, it is safer to underdilate than to overdilate the self-expanding stent. Overdilation with a high pressure squeezes the atherosclerotic material through the stent mesh, causing emboli. A 10% to 15% remaining stenosis does not cause hemodynamic problems. The self-expanding stents have a tendency for late, progressive expansion, especially if oversized (19). Importantly, it is not necessary to dilate the stent to obliterate segments of contrast-filled ulcerations or parts of the bulb external to the stent. This angiographic appearance is of no prognostic significance, and follow-up angiography has documented complete healing of these lesions over time (6). Inflation of the balloon across the distal end of the stent can cause dissection within the internal carotid artery (two occurrences with our materials; in both cases, the dissection was covered by an additional stent). If the distal end of the stent is folded inwardly and needs to be adjusted to the vessel wall, the inflation of the appropriately sized balloon has to be “soft” with no more than 1 to 2 atm. As already pointed out, it is important to keep the tip of the wire through the neck segment of the internal carotid artery up to the skull base throughout the entire procedure; the dissection can occlude the internal carotid artery. Pronounced spasm can also mimic dissection. Significant brady arrhythmias are not uncommon during predilation and stent postdilation, especially when the stenosis is ostial or within the bulb of the internal carotid artery. Bradycardia and asystole usually recover spontaneously after balloon deflation; however, occasionally, 0.5 to 1 mg of atropine is required for correction. All our patients, with one exception, responded to pharmacologic correction of bradycardia and hypotension. None had to be admitted to the intensive care unit for this reason. More prolonged bradycardia and hypotension were associated with balloon-expandable stents that place more sustained pressure on the receptors, especially when bilateral stenting was undertaken during the same procedure. No other arrhythmias were observed. We do not routinely use atropine for premedication because most of our patients are elderly and have coronary heart disease. Initially in our experience, when using balloon-expandable stents, transvenous pacemaker wires were routinely placed in the right ventricle. We have since abandoned this practice. Despite rare occurrence of significant, prolonged bradycardia, external or internal cardiac pacing has to be available. A modest fall in blood pressure requires no specific intervention. Occasionally, spasm can develop in the internal carotid artery (Fig 1B), especially after placement of the 0.018-inch wire with straightening of the artery or after stent placement. This condition can be treated with intraarterial nitroglycerin (100–200 μmg) or regresses with time.
Loss of consciousness can occur with balloon inflation, especially if the ipsilateral hemispheric blood supply is isolated or if the contralateral carotid artery is occluded. This phenomenon recovers spontaneously after immediate balloon deflation (20).
CAS has rapidly evolved into a less invasive procedure than CEA to treat patients with symptomatic and significant asymptomatic carotid stenosis. With today's equipment and techniques, stenting mortality and morbidity rates associated with stroke-related complications are in the range of 3%, similar or lower to those of comparable patient populations treated with traditional CEA (21–23), and there are no cranial nerve palsies. With both percutaneous stenting and endarterectomy, embolic stroke events can occur. With each technique, the incidence of ischemic or embolic stroke depends on meticulous procedural technique and expertise and is markedly dependent on the volume of cases performed. With the availability of dedicated carotid stenting equipment (low-profile stent delivery systems with a variety of different stent designs, better access sheaths, and specially designed wires and balloons) and carotid neuroprotective devices, current CAS results will likely be enhanced. The relatively straightforward endoluminal approach lends itself to the use of distal protection devices designed to minimize the risk of cerebral embolization. Two approaches are presently under investigation. The first, proposed by Vitek et al (24) and pioneered by Theron et al (4), involves the use of a distal occlusion balloon (4, 24, 25). The column of blood containing embolic particles within the proximal occluded internal carotid artery is aspirated. The second approach involves the deployment of a filter, which is removed after completion of CAS. The filter device seems more physiological; it provides constant cerebral perfusion. Carotid neuroprotective devices have the potential to enhance the safety of carotid stenting greatly.
Carotid angioplasty with stenting: Thirty day events (Sept 94–Sept 98)
Footnotes
Presented at the ASNR Meeting, May 26–28, 1999, San Diego, CA
Address reprint requests to Jiri J. Vitek, MD, PhD, 1520 York Avenue, Apartment 25H, New York, NY 10028.
References
- 1.Mathias K. Stent placement in supra-aortic artery disease. In: Liermann DD, ed. Stents: State of the Art and Future Developments. Morin Heights: Polyscience Publication, Inc. 1995;87-92
- 2.Diethrich EB, Ndiaye M, Reid DB. Stenting in the carotid artery: initial experience in 110 patients. J Endovasc Surg 1996;3:42-62 [DOI] [PubMed] [Google Scholar]
- 3.Roubin GS, Yadav JS, Iyer SS, Vitek JJ. Carotid stent supported angioplasty: a neurovascular intervention to prevent stroke. Am J Cardiol 1996;78:8-12 [DOI] [PubMed] [Google Scholar]
- 4.Théron JG, Payelle GG, Coskun O, Huet HF, Guimaraens L. Carotid artery stenosis: treatment with protected balloon angioplasty and stent placement. Radiology 1996;201:627-636 [DOI] [PubMed] [Google Scholar]
- 5.Wholey MH, Wholey M, Bergeron P, et al. Current global status of carotid artery stent placement. Cath Cardiovasc Diagn 1998;44:1-6 [DOI] [PubMed] [Google Scholar]
- 6.Vitek JJ, Iyer SS, Roubin GS. Carotid stenting in 350 vessels: problems faced and solved. J Invasive Cardiol 1998;10:311-314 [PubMed] [Google Scholar]
- 7. North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high grade carotid stenosis. New Engl J Med 1991;325:445-453 [DOI] [PubMed] [Google Scholar]
- 8.Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis: North American Symptomatic Carotid Endarterectomy Trial Collaborators. New Engl J Med 1998;339:1415-1425 [DOI] [PubMed] [Google Scholar]
- 9.Mihale J, Spalek P, Brozman M. So-called atheromatous pseudo-occlusion of the internal carotid atery, some diagnostic aspects. Cesk Neurol Neurochir 1985;48:316-317 [Google Scholar]
- 10. Executive Committee for the Asymptomatic Carotid Atherosclerotic Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA 1995;273:1421-1428 [PubMed] [Google Scholar]
- 11.Al-Mubarak N, Roubin GS, Gomez CR, et al. Carotid stenting in patients with high neurological risk. Am J Cardiol 1999;83:1411-1413 [DOI] [PubMed] [Google Scholar]
- 12.Gomez CR, Roubin GS, Dean LS, et al. Neurological monitoring during carotid artery stenting: the Duck Squeezing Test. J Endovasc Surg 1999;6:332-336 [DOI] [PubMed] [Google Scholar]
- 13.Mathur A, Dorros G, Iyer SS, Vitek JJ, Yadav SS, Roubin GS. Palmaz stent compression in patients following carotid artery stenting. Cath Cardiovasc Diagn 1997;41:137-140 [DOI] [PubMed] [Google Scholar]
- 14.New G, Roubin GS, Iyer SS, et al. Outpatient carotid artery stenting: a case report. J Endovasc Surg 1999;6:316-318 [DOI] [PubMed] [Google Scholar]
- 15.Mathur A, Roubin GS, Iyer SS, et al. Predictors of stroke complicating carotid artery stenting. Circulation 1998;7:1239-1245 [DOI] [PubMed] [Google Scholar]
- 16.Al-Mubarak N, Roubin GS, Vitek J, Gomez CR. Simultaneous carotid artery stenting for restenosis after endarterectomy. Cath Cardiovasc Diagn 1998;45:11-15 [DOI] [PubMed] [Google Scholar]
- 17.Al-Mubarak N, Roubin GS, Iyer SS, Gomez CR, Liu MW, Vitek J. Elective carotid artery stenting for radiation-induced carotid artery occlusive disease. J Endovasc Ther 2000;7:36-40 [DOI] [PubMed] [Google Scholar]
- 18.Ohki T, Marin ML, Lyon RT, et al. Ex vivo human carotid artery bifurcation stenting: correlation of lesion characteristics with embolic potential. J Vasc Surg 1998;27:463-471 [DOI] [PubMed] [Google Scholar]
- 19.Piamsomboon C, Roubin GS, Liu MW, et al. Relationship between oversizing of self-expanding stents and late loss index in carotid stenting. Cath Cardiovasc Diagn 1998;45:139-143 [DOI] [PubMed] [Google Scholar]
- 20.Mathur A, Roubin GS, Gomez CR, et al. Elective carotid artery stenting in the presence of contralateral occlusion. Am J Cardiol 1998;81:1315-1317 [DOI] [PubMed] [Google Scholar]
- 21.McCrory DC, Goldstein LB, Samsa GP, et al. Predicting complications of carotid endarterectomy. Stroke 1993;24:1285-1291 [DOI] [PubMed] [Google Scholar]
- 22.Rothwell PM, Slattery J, Warlow CP. A systematic comparison of the risks of stroke and death due to carotid endarterectomy for symptomatic and asymptomatic stenosis. Stroke 1996;27:266-269 [DOI] [PubMed] [Google Scholar]
- 23.Wennberg DE, Lucas FL, Birkmeyer JD, Bredenberg CE, Fisher ES. Variation in carotid endarterectomy mortality in the Medicare population. JAMA 1998;279:1278-1281 [DOI] [PubMed] [Google Scholar]
- 24.Vitek JJ, Raymon BC, Oh SJ. Innominate artery angioplasty. AJNR Am J Neuroraiol 1984;5:113-114 [PMC free article] [PubMed] [Google Scholar]
- 25.Henry M, Amor M, Henry I, et al. Carotid stenting with cerebral protection: first clinical experience using the PercuSurge guard wire system. J Endovasc Surg 1999;6:321-331 [DOI] [PubMed] [Google Scholar]


