Abstract
BACKGROUND AND PURPOSE: Rathke's cleft cysts often may be difficult to differentiate from other intrasellar or suprasellar masses on radiologic studies. The purpose of this study was to describe the significance of intracystic nodules, a diagnostic characteristic found in Rathke's cleft cysts, on MR images.
METHODS: A retrospective review of MR studies was conducted for 13 patients who, after pathologic analysis, were diagnosed as having Rathke's cleft cyst. These patients underwent unenhanced and contrast-enhanced T1- and T2-weighted axial and coronal spin-echo sequential imaging. The signal intensity and incidence of the intracystic nodules on T1- and T2-weighted images were analyzed. The signal intensity of the nodule was compared with that of white matter and surrounding cyst fluid. The signal intensity of cyst fluid was compared with the intraoperative appearance of the cyst fluid. Biochemical and pathologic analyses of the intracystic nodules were conducted in two cases.
RESULTS: An intracystic nodule having high signal intensity on T1-weighted images and low signal intensity on T2-weighted images was observed in 10 (77%) of the cases. At surgery, intracystic nodules were yellow, waxy, solid masses. Pathologic analysis showed this nodule to be a mucin clump. Biochemical analysis of the intracystic nodules showed cholesterol and proteins as the main constituents. In the Rathke's cleft cyst with intracystic nodules, cyst fluid revealed low signal intensity to isointensity relative to the intensity of the nodules on T1-weighted images, and isointensity to high signal intensity on T2-weighted images. Intracystic nodules were clearly visible on T2-weighted images.
CONCLUSION: Because cyst fluid of Rathke's cleft cysts shows variable intensities on MR images, the specific diagnosis is often difficult when based on MR signal intensity values alone. The presence of an intracystic nodule with characteristic signal intensities on MR images may be indicative of the diagnosis of Rathke's cleft cyst.
Rathke's cleft cysts are congenital, non-neoplastic sellar and suprasellar cysts derived from remnants of Rathke's pouch. These cysts are found during routine autopsies in 13% to 22% of cases (1). With the availability of CT and MR imaging, these lesions are more commonly diagnosed preoperatively or discovered incidentally. They are usually asymptomatic because they are not large enough to cause compression on surrounding structures. Symptoms, when present, result from compression of optic chiasm, hypothalamus, or pituitary gland, and are indistinguishable from those caused by other sellar masses, such as craniopharyngioma or pituitary adenoma. The treatment of symptomatic Rathke's cleft cyst differs from that of the more common sellar masses. Cyst aspiration and partial removal of Rathke's cleft cyst is regarded to be sufficient, and few recurrences have been reported. Preoperative differential diagnosis between these lesions is important for neurosurgeons when deciding the most appropriate therapy (2, 3).
Although CT and MR imaging findings may be helpful for differentiating these lesions from other intrasellar/suprasellar diseases, radiologic findings can be nonspecific, necessitating cyst wall biopsy to attempt to obtain a more definite diagnosis. There are several reports on MR findings of Rathke's cleft cysts. Most of them have described signal intensities of fluid in cysts but not the significance of intracystic nodules. In this article, we describe the significance of intracystic nodules seen on MR images in cases of Rathke's cleft cysts.
Methods
We performed a retrospective analysis correlating MR imaging and pathologic findings of 13 patients with pathologically confirmed Rathke's cleft cysts. There were seven female and six male patients ranging in age from 14 to 48 years. MR examinations were performed using 0.5-T (Gyroscan T5-II; Philips, Best, Netherlands) and 1.5-T (Vision; Siemens, Eralngen, Germany) imagers. Axial and coronal T1-weighted images (400−600/25−30 [TR/TE]) were obtained before and after the administration of gadopentetate dimeglumine (Magnevist; Schering, Berlin, Germany; 0.1 mmol/kg of body weight). Axial and coronal T2-weighted images (2000−2500/100−120) or turbo-T2-weighted images (3000−4000/90−100) were obtained before the administration of gadopentetate dimeglumine. Section thickness was 3 mm, with intersection spacing of 0%. A matrix size of 256 × 256 with a 20- to 24-cm field of view was used. Signal acquisitions were two.
The signal intensity and incidence of the intracystic nodules on T1- and T2-weighted images were analyzed. The signal intensity of the nodule was compared with that of white matter and surrounding cyst fluid. The signal intensity of cyst fluid was compared with the intraoperative appearance of the cyst fluid. Biochemical and pathologic analyses of the intracystic nodules were conducted in two cases.
Results
The results obtained from 13 patients with Rathke's cleft cysts are presented in Table 1. Clinical symptoms were observed in all patients. These included headache in eight patients, visual disturbance in three, infertility in one, and seizure in one. All patients underwent trans-sphenoidal resection of the Rathke's cleft cysts. The diameters of the Rathke's cleft cysts ranged from 5 × 5 to 18 × 10 mm. The location was intrasellar in seven patients and intrasellar and suprasellar in six patients.
Clinical, MR imaging, and intraoperative findings in Rathke's cleft cysts
In 10 (77%) of the 13 patients with the Rathke's cleft cysts, an intracystic nodule was identified on intraoperative-field and MR images. The nodules appeared high in signal intensity relative to white matter on T1-weighted images and low in signal intensity on T2-weighted images (Fig 1). Relative to the signal intensity of the nodules on the T1-weighted images, cyst fluid revealed low signal intensity in four cases and isointensity in the remaining six cases. In all cases with intracystic nodules, the cyst fluid appeared to have isointensity to high signal intensity relative to the signal intensity of the nodules on the T2-weighted images. Intracystic nodules were clearly visible on the T2-weighted images.
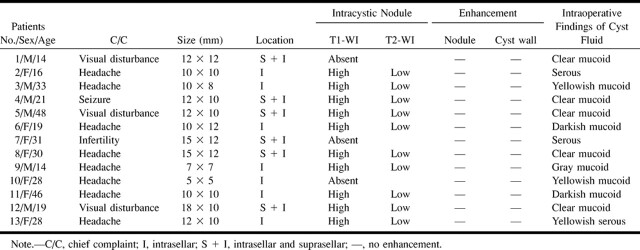
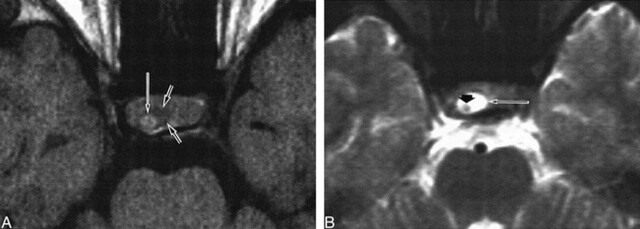
fig 1.
Patient 9. Rathke's cleft cyst with intracystic nodule.
A, Nodule (long arrow) in the cyst shows high signal intensity. Cystic fluid (small arrow) shows low signal intensity on axial T1-weighted image (600/25/2).
B, High signal intensity of surrounding fluid (long arrow) and low signal intensity of intracystic nodule (small arrow) are well delineated on axial T2-weighted image (2300/120/2).
Surgery revealed that the nodules were yellow, waxy, solid masses. Although pathologic findings of the intracystic nodules showed mucinous masses, the presence of cholesterol and protein was confirmed by biochemical analysis.
Surgery also revealed that the cystic fluid of Rathke's cleft cyst was mucoid in 10 cases and serous in three. Six of the cases with mucoid fluid had cysts with high signal intensity relative to white matter on T1-weighted images and variable intensity (two low and four high) on T2-weighted images. The remaining four cases had isointensity to low signal intensity on T1-weighted images and high signal intensity on T2-weighted images. Two cases with serous cystic fluid had high signal intensity on T1- and T2-weighted images. In another case with serous fluid, the cyst fluid was isointense to white matter on T1-weighted images and had low signal intensity on T2-weighted images.
Discussion
Rathke's cleft cysts are thought to arise from failure of obliteration of the lumen of Rathke's pouch, which develops as a rostral outpouching of the primitive oral cavity during the third or fourth week of gestation. The epithelium of Rathke's cleft cyst is a vestige of Rathke's pouch that is the precursor of the anterior lobe, intermediate lobe, and pars tuberalis of the pituitary gland. A Rathke's pouch has an anterior and a posterior wall and a central embryonic cleft. The anterior wall of the pouch proliferates to form the anterior lobe of the pituitary gland and the pars tuberalis; the posterior wall becomes the pars intermedia. The residual lumen of the pouch is reduced to a narrow Rathke's cleft, which generally regresses (4, 5). It is the persistence and enlargement of this cleft that is said to be the cause of the symptomatic Rathke's cleft cyst. Alternatively, other investigators have suggested that Rathke's cleft cyst originates directly from neuroepithelial tissue, metaplasia of anterior pituitary cells, or endoderm (6, 7).
The wall of the embryonic cleft is histologically similar to the wall of the single cell–layered Rathke's cleft cysts. Both are typically lined by a single cell layer of epithelium that is frequently ciliated and often contains goblet cells. Typical pathologic findings of Rathke's cleft cysts are tall, well-differentiated, columnar epithelium with ciliated and goblet cells, but frequently this classic lesion is altered by the presence of squamous metaplasia (8).
In general, Rathke's cleft cysts are less than 3 mm in diameter and are usually asymptomatic. Asymptomatic Rathke's cleft cysts are present in 13% to 22% of pituitary glands randomly examined at autopsy (1). Symptomatic Rathke's cleft cysts are uncommon. These cysts can enlarge and cause symptoms secondary to compression of the pituitary gland, pituitary stalk, or hypothalamus. The most common clinical manifestations of enlarged cysts include hypopituitarism, diabetes insipidus, visual disturbance, and headache (9). Ross et al (10) reported on data obtained from 43 patients with Rathke's cleft cyst treated by one neurosurgeon. They noted that headache is the most common symptom and that galactorrhea, visual field loss, and hypopituitarism are the next most common signs. The most common symptom in our study was headache.
Rathke's cleft cysts are often difficult to distinguish from cystic craniopharyngiomas or cystic pituitary adenomas on radiologic studies. Hua et al (11) analyzed the MR imaging features in nine cases of surgically confirmed non-neoplastic intra- and suprasellar cysts, such as Rathke's cleft cysts, and compared them with 17 cases of craniopharyngiomas and 12 of cystic pituitary adenomas. They concluded that the signal intensity of the cystic fluid did not help in distinguishing non-neoplastic cysts from cystic neoplasms, but enhancement of the wall of a cystic neoplasm on contrast-enhanced MR images played an essential role in differentiating neoplastic from non-neoplastic cysts. Nonetheless, non-neoplastic cysts such as Rathke's cleft cysts are often surrounded by the enhancing normal pituitary gland, thus mimicking wall enhancement. Rapid enhancement during the early phase of the administration of contrast material (dynamic studies) may help to avoid confusion between the normal pituitary tissue and cyst wall enhancement (11).
Ahmadi et al (12) reported that biochemical analysis of cystic fluid in craniopharyngiomas in 10 patients was performed to correlate signal intensity on T1-weighted MR images. They stated that high or low signal intensity of cystic fluid on T1-weighted images depends on the concentration of cystic fluid composition, such as protein, cholesterol, and triglyceride. Signal intensity of cyst fluid in our study of 13 patients was variable and not diagnostic. Because the cyst fluid of Rathke's cleft cyst, cystic craniopharyngioma, and cystic pituitary adenoma shows variable signal intensities on MR images, the diagnosis of Rathke's cleft cyst is not possible based on signal intensity of cyst fluid on MR images alone.
In our study, intracystic nodules were frequently found in Rathke's cleft cysts. In some cases, however, signal intensities of cyst fluid relative to intracystic nodules are similar, often making the detection of the intracystic nodule difficult. Therefore, most articles have overlooked the significance of the intracystic nodules in Rathke's cleft cysts.
In our results, 10 of the 13 Rathke's cleft cysts contained intracystic nodules, which showed high signal intensity on T1-weighted images and low signal intensity on T2-weighted images. In all cases, the surrounding cystic fluid had isointensity to low signal intensity relative to the intracystic nodules on T1-weighted images. Therefore, the detection of the intracystic nodules on T1-weighted images was often difficult. Because most of the intracystic nodules reveal low signal intensity relative to that of surrounding cystic fluid on T2-weighted images, intracystic nodules were well defined on T2-weighted images, especially in cases in which the cyst fluid was high in signal intensity on T1-weighted images. Enhancement of the cyst wall on contrast-enhanced MR images was not shown. Also, intracystic nodules and their margins were not enhanced.
The nodules in craniopharyngiomas are also common. Although typical craniopharyngioma is a lobulated, well-delineated, cystic mass with a mural nodule, the nodules of craniopharyngioma are hypointense on T1-weighted images, are hyperintense on T2-weighted images, and enhance strongly but heterogeneously after the administration of contrast material (13, 14). Therefore, the presence of an intracystic nodule with characteristic signal intensities on MR images may be indicative of the diagnosis of Rathke's cleft cyst.
Sumida et al (15) reported that intracystic nodules were found in three of the 18 patients with Rathke's cleft cysts, but they did not conduct biochemical analysis of the intracystic nodules. Kuwahara et al (16) reported a case of Rathke's cleft cyst with a moving mass in the cyst. They described that a brownish globular mass, 6 mm in diameter, existed within the cyst without connection to the surrounding tissue. In our study, intracystic nodules freely floated without connection by any membrane.
Kucharczyk et al (17) reported that three of the seven cases had a solid waxy component that was adherent to the cyst wall. They stated that pathologic studies showed an epithelial-lined cyst containing acellular proteinaceous material with a white nodule of adherent solid tissue that represented desquamated cellular debris. Biochemical analysis of cyst content was performed by Nemoto et al (18), who suggested that cholesterol is not hyperintense on T1-weighted images, whereas mucopolysaccharide is. Hayashi et al (19) analyzed the cyst fluid of five Rathke's cleft cysts by conducting biochemical analysis. The main constituents of cyst fluid were protein and cholesterol. They suggested that shortened T1 relaxation times depended on the very high protein concentrations in the cysts rather than on the cholesterol levels.
In our study, intracystic nodules appeared as mucinous material on histologic examination and as cholesterol and protein on biochemical analysis. Therefore, we suggest that the nodule is a concretion of material within the cyst and that the amount of protein in intracystic nodules influences MR signal intensity.
Conclusion
Rathke's cleft cysts are approached surgically in a manner different from that used for sellar and juxtasellar tumors, such as pituitary adenomas and craniopharyngiomas. Preoperative differential diagnosis between these lesions is important for neurosurgeons when deciding the most appropriate therapy. Because cyst fluid of Rathke's cleft cysts shows variable intensities on MR images, the diagnosis is often difficult when based on MR signal intensity values alone. An intracystic nodule in Rathke's cleft cyst is not uncommon. Detection of intracystic nodules with characteristic signal intensities on MR images, such as high signal intensity on T1-weighted images and low signal intensity on T2-weighted images, may be a diagnostic indicator of Rathke's cleft cyst.
Acknowledgments
We thank Jong Oh Choi, MD, and Hyun Soo Kim, MD, for invaluable assistance.
Footnotes
Address reprint requests to Woo Mok Byun, MD, Department of Diagnostic Radiology, College of Medicine, Yeungnam University, 317–1, Daemyungdong, Namku, Taegu 705–717, Korea.
References
- 1.Shanklin WM. On the presence of cysts in the human pituitary. Anat Rec 1949;104:399-407 [DOI] [PubMed] [Google Scholar]
- 2.Baskin DS, Wilson CB. Transsphenoidal treatment of non-neoplastic intrasellar cysts. J Neurosurg 1984;60:8-13 [DOI] [PubMed] [Google Scholar]
- 3.Hardy J, Vezina JL. Transsphenoidal neurosurgery of intracranial neoplasm. Adv Neurol 1976;15:261-274 [PubMed] [Google Scholar]
- 4.Barrow DL, Spector RH, Yakei Y, Tindall GT. Symptomatic Rathke cleft cysts located entirely in the suprasellar region: review of diagnosis, management, and pathogenesis. Neurosurgery 1985;16:766-772 [DOI] [PubMed] [Google Scholar]
- 5.McGrath P. Cysts of sellar and pharyngeal hypophyses. Pathology 1871;3:123-131 [DOI] [PubMed] [Google Scholar]
- 6.Shuangshoti S, Netsky MG, Nashold BSJ. Epitherial cysts related to sella turcica. Arch Pathol 1970;90:444-450 [PubMed] [Google Scholar]
- 7.Rasmussen AT. Ciliated epithelium and mucus secreting cells in the human hypophysis. Anat Rec 1929;41:273-283 [Google Scholar]
- 8.Burger PC, Scheithauer BW. Benign cystic lesions. In: Rosai J, ed. Tumors of the Central Nervous System. Ed 1. Washington: AFIP; 1993;355-370
- 9.Yoshida J, Kobayashi T, Kageyama N, Kanzaki M. Symptomatic Rathke's cleft cyst: morphological study with light and electron microscopy and tissue culture. J Neurosurg 1977;47:451-458 [DOI] [PubMed] [Google Scholar]
- 10.Ross DA, Norman D, Wilson CB. Radiologic characteristics and results of surgical management of Rathke's cysts in 43 patients. Neurosurgery 1992;30:173-179 [DOI] [PubMed] [Google Scholar]
- 11.Hua F, Asato R, Miki Y, et al. Differentiation of suprasellar nonneoplastic cysts from cystic neoplasms by Gd-DTPA MRI. J Comput Assist Tomogr 1992;16:744-749 [DOI] [PubMed] [Google Scholar]
- 12.Ahmadi J, Dstian S, Apuzzo MLJ, Segall HD, Zee CS. Cystic fluid in craniopharyngioma: MR imaging and quantatative analysis. Radiology 1992;182:783-785 [DOI] [PubMed] [Google Scholar]
- 13.Hald JK, Eldevik OP, Skalpe IO. Craniopharyngioma identified by CT and MR imaging at 1.5T. Acta Radiologica 1995;36:142-147 [PubMed] [Google Scholar]
- 14.Osborn AG. Miscellaneous tumors, cysts, and metastases. In: Patterson AS, ed. Diagnositic Neuroradiology. Ed 1. St.Louis: Mosby; 1994;626-670
- 15.Sumida M, Uozumi T, Mukada K, Arita K, Kurisu K, Eguchi K. Rathke cleft cysts: correlation of enhanced MR and surgical findings. AJNR Am J Neuroradiol 1994;15:525-532 [PMC free article] [PubMed] [Google Scholar]
- 16.Kuwahara T, Shinohara Y, Sugiura M, Hiramatsu H. A Rathke's cleft cyst with a moving mass in the cyst. No Shinkei Geka 1997;25:177-180 [PubMed] [Google Scholar]
- 17.Kucharczyk W, Peck WW, Kelly WM, Norman D, Newton TH. Rathke cleft cysts: CT, MR imaging, and pathologic features. Radiology 1987;165:491-495 [DOI] [PubMed] [Google Scholar]
- 18.Nemoto Y, Inoue Y, Fukuda T, et al. MR appearance of Rathke's cleft cysts. Neuroradiology 1988;30:155-159 [DOI] [PubMed] [Google Scholar]
- 19.Hayashi Y, Tachibana O, Muramatsu N, et al. Rathke cleft cyst: MR and biomedical analysis of cyst content. J Comput Assist Tomogr 1999;23:34-38 [DOI] [PubMed] [Google Scholar]