Scheme 1. Scope of the Annulation of N,N-Diethyl-o-Toluamides with Cyclic Imines Generated in Situ and Applications.
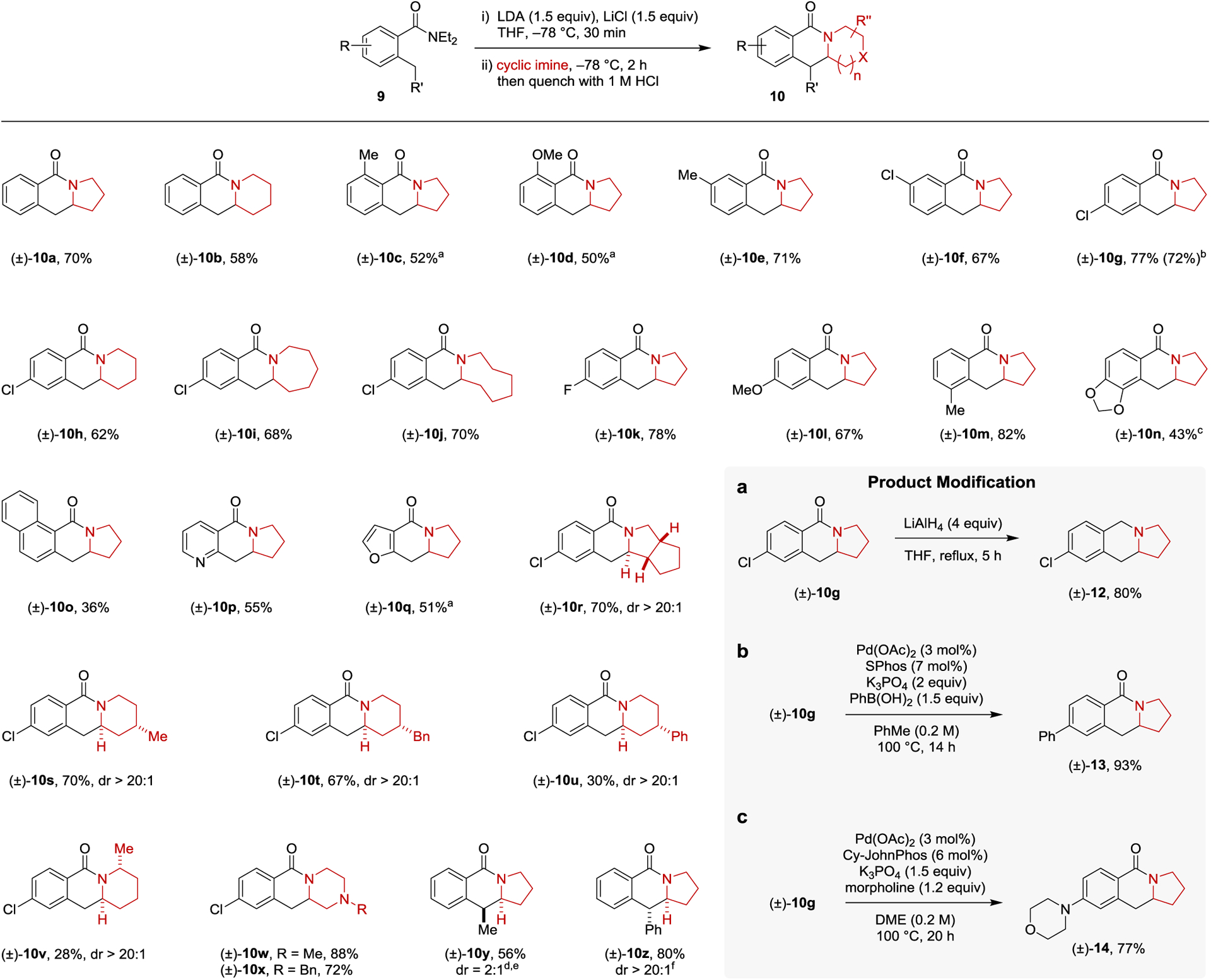
Reactions were performed with 0.5 mmol of 9. Yields correspond to isolated yields. THF (2.5 mL) was used for the lithiation of 9. Cyclic imines were prepared in situ by adding n-BuLi (2 equiv) to a solution of the corresponding cyclic amine (2 equiv) in ether (1 mL) at −78 °C, followed by the addition of trifluoroacetophenone (2 equiv). a o-Toluamide lithiation was performed at −40 °C. b Yield in parenthesis corresponds to reaction conducted on a 2 mmol scale. c o-Toluamide lithiation was performed using s-BuLi/TMEDA (1.5 equiv each) at −78 °C for 30 min. d o-Toluamide lithiation was performed using s-BuLi/TMEDA (2 equiv each) at −78°C for 1 h. e 3 Equiv of 1-pyrroline was used. f Reaction was warmed up to room temperature over 30 min after 1-pyrroline was added. Product Modification a, Reduction of lactam to amine. b, Suzuki-Miyaura coupling. c, Buchwald-Hartwig coupling.
