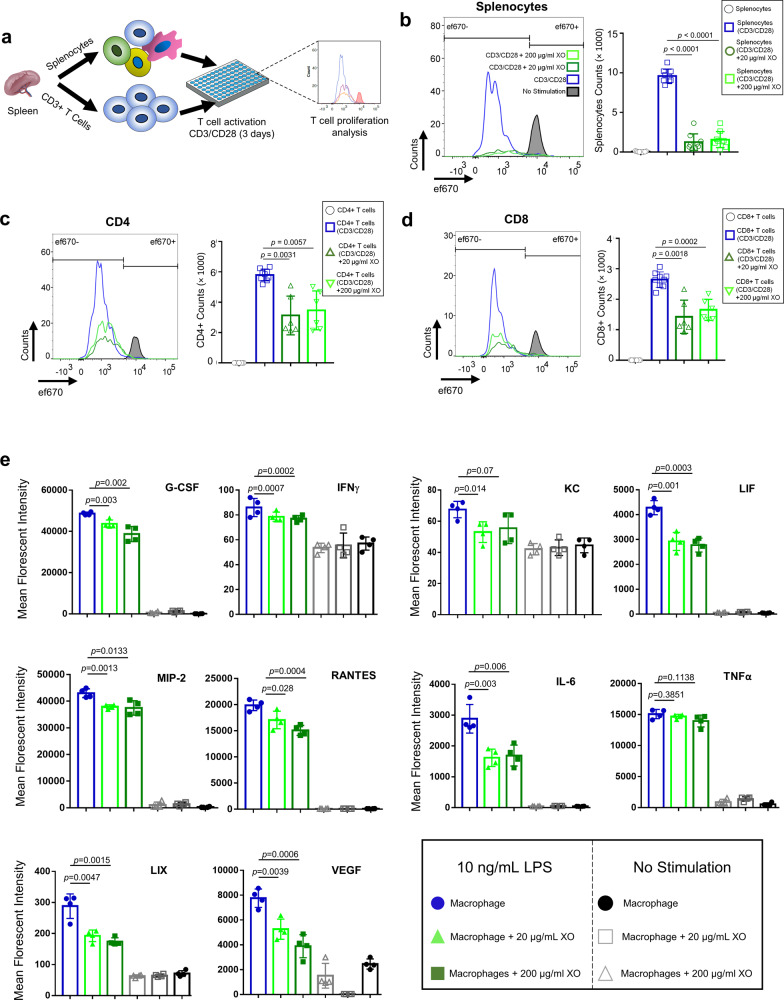
Fig. 4. XOs suppress the proliferation of splenocytes and CD3+ T cells and reduce the production of inflammatory cytokines from LPS-stimulated macrophages.
a Schematic figure showing the experimental procedure. CFSE-labeled splenocytes and CD3+ T cells were co-cultured with plate-bound anti-CD3 and soluble CD28 in the presence or absence of 20 and 200 μg/mL of XOs. Upon 4 days co-culture, cells were analyzed using flow cytometry. b Splenocyte counts for CD3/CD28 activated cells were 9603 ± 871, and addition of 20 and 200 μg/mL XOs reduced the counts to 1253 ± 1038 (n = 4, p < 0.0001, 4 mice) and 1570 ± 1010 (n = 4, p < 0.0001), respectively. c In the co-cultures of CD3+ cells with CD3/CD28 antibodies, CD4+ counts for CD3/CD28 activated T cells was 5217 ± 378. Addition of 20 and 200 μg/mL XOs reduced the counts to 3889 ± 2081 (n = 4, p = 0.0031, 4 mice) and 4387 ± 1397 (n = 4, p = 0.0057, 4 mice), respectively. d In the co-cultures of CD3+ cells with CD3/CD28 antibodies, CD8+ counts for CD3/CD28 activated T cells was 2700 ± 252. Addition of 20 and 200 μg/mL XOs reduced the counts to 1503 ± 784 (n = 4, p = 0.0018, 4 mice) and 1766 ± 628 (n = 4, p = 0.0002), respectively. e Addition of XOs to the co-cultures of murine macrophages reduces the secretion of inflammatory cytokines (G-CSF, IFNγ, IL-6, LIF, LIX, MIP-2, RANTES) in a dose-dependent manner (n = 4, biological replicates). Results are mean ± SD, and statistical significance is calculated through unpaired t-test with Welch’s correction.