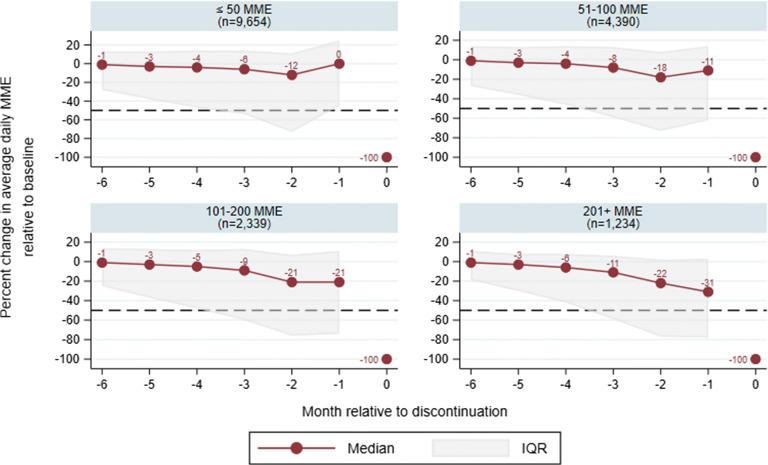
Figure 1.
Relative change in daily MME dose compared to baseline period, by average daily MME at baseline (7 to 12 months before discontinuation). Each panel is labeled with the average daily MME group at baseline, and the total numbers of LTOT users in each group are in parentheses. The black dashed line demarcates a threshold of 50% of the baseline daily MME dose. Patients whose dose is above this by month − 1 are classified as having an “abrupt” discontinuation, whereas those below have a “> 50% taper” that could be consistent with guidelines. While our sample excludes beneficiaries with less than 25 average daily MME in the initial 12-month LTOT episode, it is possible for beneficiaries who discontinued LTOT to have less than 25 average daily MME in the 7 to 12 months before discontinuation; therefore, the lowest dose category “≤ 50 MME” captures many beneficiaries with discontinuation.