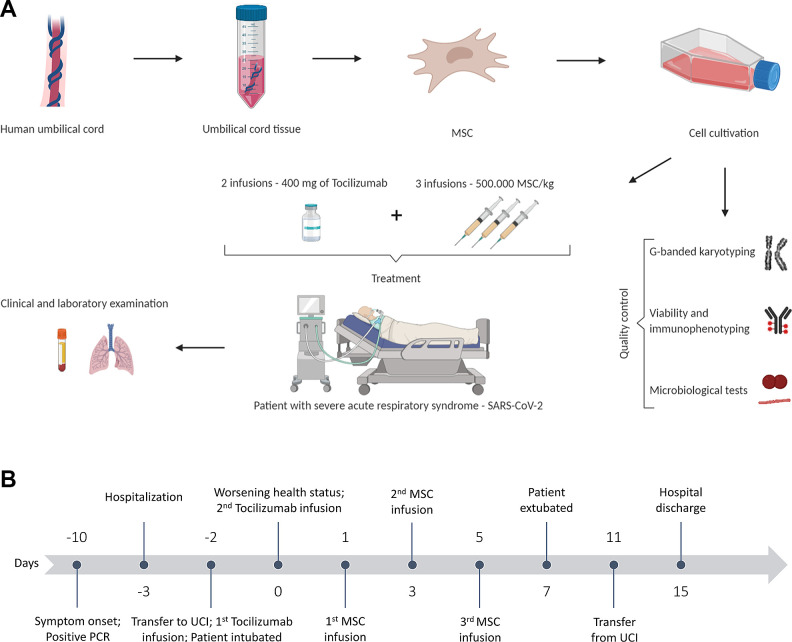
Fig. 1.
Study design. (A) Umbilical cord-derived mesenchymal stromal cells (UC-MSC) were isolated, expanded, and evaluated for surface markers, viability, presence of fungi and bacteria, and chromosomal abnormalities. The patient received three infusions of 500.00 UC-MSCs/kg and two infusions of 400 mg of tocilizumab. The patient’s clinical and laboratory evaluations were performed pre-MSC infusion (D1) and on days 2, 4, 6, 14, 60, and 120. (B) Schematic representation of the days before (minus) and after the first infusion of UC-MSC.