Abstract
Botulism is caused by a potent neurotoxin that blocks neuromuscular transmission, resulting in death by asphyxiation. Currently, the therapeutic options are limited and there is no antidote. Here, we harness the structural and trafficking properties of an atoxic derivative of botulinum neurotoxin (BoNT) to transport a function-blocking single-domain antibody into the neuronal cytosol where it can inhibit BoNT serotype A (BoNT/A1) molecular toxicity. Post-symptomatic treatment relieved toxic signs of botulism and rescued mice, guinea pigs, and nonhuman primates after lethal BoNT/A1 challenge. These data demonstrate that atoxic BoNT derivatives can be harnessed to deliver therapeutic protein moieties to the neuronal cytoplasm where they bind and neutralize intracellular targets in experimental models. The generalizability of this platform might enable delivery of antibodies and other protein-based therapeutics to previously inaccessible intraneuronal targets.
INTRODUCTION
Botulinum neurotoxins (BoNTs), produced by the anaerobic bacterium Clostridium botulinum and related species, are among the most potent known toxins (1-3). With estimated human lethal doses in the range of 1 ng/kg and the capacity to intoxicate by aerosol and oral routes, a low amount can have lethal consequences for a large number of individuals (4, 5). BoNTs are serologically divided into seven classical serotypes (designated A to G), many of which contain multiple subtypes (6). Multiple new variants have also been recently reported, some of which may ultimately be categorized as new serotypes (7-9). All BoNTs are synthesized as single-chain proproteins, which are converted to the active heterodimer by endogenous bacterial proteases (BoNTs produced by Clostridium argentinense or Clostridium botulinum groups I and III) or by exogenous enzymes after secretion or ingestion (BoNTs produced by C. botulinum group II) (10). Enzymatic activation converts the proprotein into a heterodimer consisting of a light chain (LC; ~50 kDa) and a heavy chain (HC; ~100 kDa; Fig. 1A) linked by a single disulfide bond. The HC mediates highly specific binding of the heterodimer to neuronal presynaptic plasma membrane receptors, internalization into endocytic vesicles, and translocation of the LC into the neuronal cytoplasm, where the LC metalloprotease proteolytically cleaves soluble N-ethylmaleimide–sensitive factor attachment protein receptor (SNARE) proteins: synaptosomal–associated protein 25 (SNAP-25), syntaxin 1 (STX1), or vesicle-associated membrane protein 1-3 (VAMP1-3) (11). This last step prevents functional assembly of the neuronal machinery required for neuromuscular transmission, causing the flaccid paralysis characteristic of clinical botulism.
Fig. 1. Design of B8C1ad and inhibition of BoNT/A1 in vitro.
(A) BoNTs have a highly conserved structure, consisting of a disulfide-linked heterodimer with modular functional domains. (B) B8C1ad consists of the B8 sdAb fused to the N terminus of atoxic C1ad. C1ad carries three point mutations in LC (E425>A; H428>G; Y570>A) that eliminate metalloprotease activity. TD, translocation domain; RBD, receptor-binding domain; HC, heavy chain; B8LC fusion of sdAb B8 with LC/C1ad; and S-S, disulfide bridge connecting B8LC and HC. OLLAS (O), c-myc (M), E-tag (E), and HA (H) epitope tags. (C) Effects of B8C1ad and C1ad on LC/A1 proteolytic activity in a FRET-based substrate cleavage assay. FRET ratios were normalized to positive and negative control reactions: 0% corresponds to the FRET ratio without BoNT/A1, and 100% corresponds to the FRET ratio with BoNT/A1 and 10 mM DTT.
The current treatment for botulism is post-exposure prophylaxis with equine-derived heptavalent botulinum antitoxin (HBAT) combined with chronic ventilation and supportive care as needed. HBAT neutralizes toxin in the bloodstream but is ineffective once toxin has bound to or been internalized into neurons (11). To reduce the risks of anaphylaxis, HBAT use is limited to patients with a confirmed BoNT exposure. Early botulism symptoms are nonspecific, and thus, HBAT administration is often delayed pending differential diagnosis (12). This delay is clinically important because the majority of patients that develop botulism symptoms ultimately require intubation and ventilation for survival (13, 14). Intensive care facilities have limited capacity to accommodate a surge of patients, and thus, even small outbreaks of botulism can strain or exhaust the medical resources of major metropolitan areas (2). Furthermore, some BoNT serotypes cause paralysis that lasts for weeks or longer (13, 14), during which time ventilated patients remain at elevated risk of dangerous comorbidities.
The stark limitations of current botulism treatments have necessitated a search for pharmacotherapies that accelerate symptomatic reversal. In particular, considerable effort has been directed to the discovery and development of small molecules that inhibit the LC metalloprotease activity (4, 15, 16). Given the acute toxicity of BoNTs and the persistent ability of LC and/or cleaved SNARE protein to antagonize neurotransmission, it is apparent that LC inhibitors must be administered as early as possible to prevent accumulation of cleaved SNARE proteins in the presynaptic compartment and concomitant toxic effects on neurotransmission. Although these efforts have identified small molecules that potently block LC activity in solution, efficacy in vivo has not yet been demonstrated.
We recently described the rational design of a BoNT serotype C1 (BoNT/C1) mutant containing three point mutations that eliminate LC metalloprotease activity. The resulting atoxic derivative (C1ad) retains the neuronal targeting and intracellular trafficking of BoNT/C1, yet has 106-fold lower toxicity in vivo (17). Hypothesizing that C1ad could be used to transport therapeutic proteins into the neuronal cytosol, we developed and tested a potential treatment for botulism based on intracellular inhibition of the BoNT/A1 LC metalloprotease (LC/A1). Here, we show that a precision biotherapeutic consisting of a function-blocking single-domain antibody (sdAb; B8) cargo (18) fused to the C1ad delivery vehicle (forming B8C1ad; Fig. 1, A and B) can enter neurons and protect SNARE proteins by inhibiting LC/A1 catalytic activity in situ. Post-symptomatic administration of B8C1ad produced antidotal rescue in mice, guinea pigs, and nonhuman primates after a lethal botulism challenge.
RESULTS
Bioengineering and in vitro validation of B8C1ad
The therapeutic protein B8C1ad was created by genetically fusing the sequence of the B8 sdAb to the N terminus of C1ad (Fig. 1, A and B). The B8 sdAb was chosen because it selectively binds dissociated LC/A1 but not BoNT/A1 holotoxin (fig. S1). Thus, B8 sdAb recognizes LC/A1 only after it has separated from the HC, such as after translocation into the neuronal cytosol. B8C1ad was expressed as a proprotein, purified, and converted to the active heterodimer form by digestion at engineered tobacco etch virus (TEV) cleavage sites (17). Sequence integrity of the activated heterodimer was verified by mass spectrometry and immunodetection (figs. S2 and S3). To confirm that the function-blocking properties of B8 were not impaired by fusion to C1ad, LC/A1 catalytic activity was measured in the presence of B8C1ad or C1ad in a fluorescence resonance energy transfer (FRET)–based cleavage assay. Compared to C1ad, which did not protect the target substrate from cleavage, B8C1ad inhibited LC/A1 at equimolar and higher concentrations (P = 0.0001 versus C1ad at each concentration; Fig. 1C).
B8C1ad blocks BoNT/A1 molecular toxicity in primary neuronal cultures
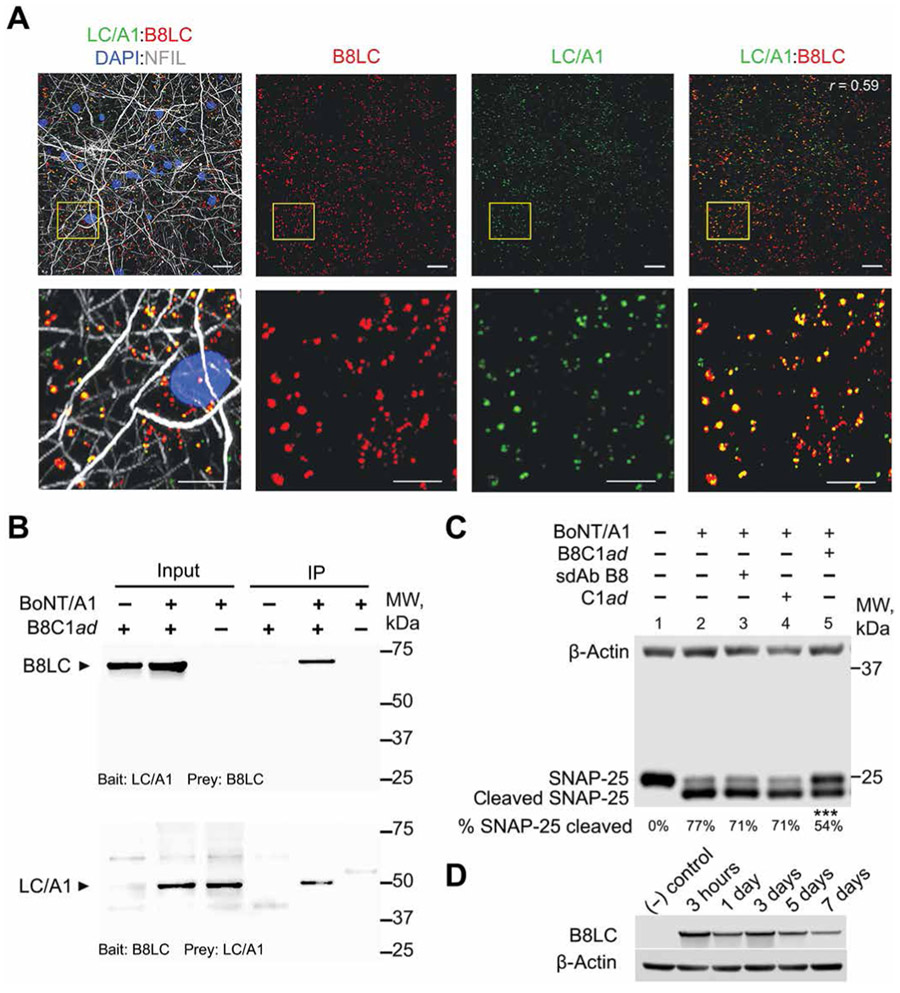
In principle, treatment of neurons with B8C1ad should result in the translocation of B8LC into the neuronal cytosol. To establish whether B8C1ad retained the neuronal uptake and intracellular trafficking properties of wild-type BoNT, intracellular localization of B8LC was evaluated in primary rat cortical neurons treated with B8C1ad for 24 hours followed by a 24-hour chase with fresh medium. Fluorescent colocalization studies revealed colocalization of B8LC with STX1 (r = 0.81, n = 3) and SNAP-25 (r = 0.36, n = 3) in the presynaptic compartment (fig. S4) but not with endosomal (r = −0.1, n = 3), lysosomal (r = 0.1, n = 3), or autophagosomal markers (r = −0.1, n = 3; fig. S5), consistent with presynaptic uptake and B8LC translocation into the cytosol. Neurons intoxicated with BoNT/A1 and chased with B8C1ad showed strong intracellular colocalization of B8LC and LC/A1 (r = 0.59, n = 3; Fig. 2A). B8LC and its LC/A1 target were reciprocally coimmunoprecipitated from treated neurons, confirming the interaction between B8LC and LC/A1 in the neuronal cytosol (Fig. 2B).
Fig. 2. B8C1ad protects SNAP-25 against BoNT/A1 metalloprotease activity in rat cortical neurons.
Rat E18 cortical neurons were cultured for 14 days before treatments. (A) Representative fluorescent images showing colocalization of LCA1 with B8LC after sequential treatment of primary neuron cultures with BoNT/A1 (670 pM; 2 hours), followed by 2-hour chase with fresh medium, followed by B8C1ad (10 nM; 16 hours). Bottom panels are higher-magnification views of region marked in yellow. Scale bars, 10 μm (top images) and 2 μm (bottom images). Pearson’s correlation for colocalization (r) is presented in the top right panel. (B) Representative Western blots showing reciprocal immunoprecipitation (IP) of B8LC and LC/A1 from neurons exposed to BoNT/A1 (6 nM; 24 hours) and chased with fresh medium for 2 hours, followed by incubation with B8C1ad (50 nM; 24 hours). Coimmunoprecipitation bait and prey combinations are presented at the bottom of each Western blot. (C) Representative Western blot showing SNAP-25 cleavage in lysates from primary neurons exposed to 5 pM BoNT/A1 for 24 hours, washed, chased with fresh medium for 2 hours, and treated with 50 nM B8C1ad, 50 nM sdAb B8, 50 nM C1ad, or vehicle for 24 hours. The percentage of cleaved SNAP-25 is shown below each lane (N = 5, n = 3 to 5 for each condition). (D) Representative Western blot showing persistence of B8LC in neurons incubated with 50 nM B8C1ad for 48 hours, and then washed and cultured for an additional 3 hours or 1, 3, 5, or 7 days before analysis. β-Actin is used as a loading control in (C) and (D). Additional details of statistical comparisons are presented in table S3. ***P < 0.001.
To directly address whether B8C1ad mitigates BoNT/A1 toxicity in a post-exposure model, neurons were intoxicated with BoNT/A1 for 24 hours, chased with fresh medium for 2 hours, and treated with B8C1ad or vehicle. Compared to vehicle, treatment with B8C1ad significantly reduced SNAP-25 cleavage (vehicle: 76.6 ± 4.5%, n = 5; B8C1ad: 54.3 ± 3.0%, n = 3; P = 0.0006), whereas sdAb B8 (70.5 ± 0.59%, n = 3; P = 0.49) or C1ad (70.7 ± 3.8%, n = 4; P = 0.33) did not prevent SNAP-25 cleavage (Fig. 2C). To evaluate the duration of B8C1ad therapeutic effects, neurons were incubated with 5 pM BoNT/A1 for 1.5 hours, chased with fresh medium for 2 hours, incubated with 50 nM B8C1ad or vehicle for 24 hours, and analyzed for SNAP-25 cleavage after 1, 4, or 7 days. Compared to vehicle, B8C1ad reduced SNAP-25 cleavage at 1 day (B8C1ad: 19.3 ± 6.6% versus vehicle: 49.3 ± 2.0%, n = 3 each; P = 0.019), 4 days (B8C1ad: 54.3 ± 4.6% versus vehicle: 75.7 ± 4.3%, n = 3 each; P = 0.014), and 7 days (B8C1ad: 52.3 ± 5.9% versus vehicle: 78.3 ± 5.6%, n = 3 each; P = 0.007; fig. S6A). After confirming that B8LC persisted in neuronal cultures for at least 7 days (Fig. 2D), we found that pretreatment of primary neuronal cultures with B8C1ad for 1 day protected against SNAP-25 cleavage compared to vehicle when neurons were subsequently intoxicated with BoNT/A1 at 2 days (B8C1ad: 36.3 ± 3.5% versus vehicle: 57.3 ± 3.5%, n = 3 each; P = 0.013), 4 days (B8C1ad: 36.3 ± 4.4% versus vehicle: 59.0 ± 4.0%, n = 3 each; P = 0.020), or 9 days (B8C1ad: 49.0 ± 2.9% versus vehicle: 64.7 ± 3.8%, n = 3 each; P = 0.033; fig. S6B). The ability of B8C1ad to protect against SNAP-25 cleavage both in pre- and post-intoxication models excluded the possibility that B8C1ad interfered with BoNT/A1 neuronal uptake or translocation. Together, these data suggest that B8C1ad treatment blocks BoNT/A1 molecular toxicity through intraneuronal inhibition of LC/A1.
Therapeutic efficacy of B8C1ad in a murine model of lethal BoNT/A1 botulism
We next tested B8C1ad as a post-symptomatic treatment in mice after lethal BoNT/A1 challenge. To ascertain the maximum therapeutic dose, the acute toxicity of B8C1ad was determined in dose-ranging studies (Fig. 3A). The intraperitoneal median lethal dose (LD50) of B8C1ad was estimated to be 1.45 ± 0.05 mg/kg, with a no observed adverse effect level (NOAEL) of 0.4 mg/kg (table S1). Thus, disabling LC metalloprotease activity reduces the acute toxicity of B8C1ad more than 107-fold versus BoNT/A1 (Fig. 3A) and 106-fold versus wild-type BoNT/C1 (17).
Fig. 3. B8C1ad antidotal rescue in mice after lethal BoNT challenge.
(A) Mouse intraperitoneal (i.p.) LD50 values for B8C1ad (n = 12 mice per dose, seven doses) and BoNT/A1 (n = 5 mice per dose, eight doses). (B) Ten-day survival in mice intoxicated with BoNT/A1 (2 mipLD50; intraperitoneally) and treated 5.5 hours later by intraperitoneal injection of vehicle (n = 15), B8C1ad (0.4 mg/kg; n = 15), sdAb B8 (0.35 mg/kg; n = 10), or C1ad (0.04 mg/kg; n = 10). (C) Ten-day survival in mice challenged with 4 and 6 mipLD50 BoNT/A1 (intraperitoneally) and treated 12 hours later with vehicle (intraperitoneally; n = 5 per group) or B8C1ad (0.4 mg/kg, intraperitoneally; n = 5 per group). (D) Ten-day survival in mice pretreated with B8C1ad (0.4 mg/kg, intraperitoneally) and challenged with BoNT/A1 (2 mipLD50, intraperitoneally) after 2 days (n = 12), 3 days (n = 10), or 4 days (n = 10). The vehicle group was treated 2 days before challenge (n = 10). All data represent the aggregate of at least three experiments. Additional details of statistical comparisons are presented in table S3. CI, confidence interval. *P < 0.5, **P < 0.01, and ****P < 0.0001.
Control mice intoxicated with two mouse intraperitoneal LD50 (mipLD50) of BoNT/A1 exhibited mild abdominal paradox by 8 hours (range, 6 to 8 hours) with a median survival time of 27 hours (range, 22 to 34 hours; table S2). To test B8C1ad in a post-symptomatic treatment model, mice were challenged with 2 mipLD50 BoNT/A1 and treated 12 hours later with vehicle, B8C1ad, or equimolar doses of sdAb B8 or C1ad. All mice treated with vehicle, B8 sdAb, or C1ad expired within 2 days, whereas B8C1ad significantly improved 10-day survival to 93.3% (14 of 15; P < 0.0001; Fig. 3B). Surviving mice were alert and responsive at 10 days. To test B8C1ad efficacy at the upper bounds of a typical foodborne exposure (19, 20), mice were challenged with 4 or 6 mipLD50 BoNT/A1 and treated 5.5 hours later with vehicle or B8C1ad (Fig. 3C). All mice exhibited respiratory signs of botulism at time of treatment. Compared to vehicle, B8C1ad increased 10-day survival at 4 mipLD50 (vehicle: 0 of 5 versus B8C1ad: 3 of 5; P = 0.17) and prolonged median survival time at 6 mipLD50 (vehicle: 1 day versus B8C1ad: 4 days; n = 5 each, P = 0.014), demonstrating therapeutic efficacy across the expected dose range for natural botulism exposures.
Because (i) pretreatment of cultured neurons with B8C1ad reduced SNAP-25 cleavage by BoNT/A1 (fig. S6B) and (ii) B8LC exhibited prolonged persistence in the nerve cytosol (Fig. 2D), we hypothesized that B8C1ad would have prophylactic effects in vivo. Mice were given B8C1ad (n = 10 to 12) or vehicle (n = 10) intraperitoneally and challenged 2, 3, or 4 days later with 2 mipLD50 BoNT/A1 (Fig. 3D and table S3). Compared to vehicle (0 of 10 survivors), survival rates were improved by a 1-day pretreatment with B8C1ad when mice were intoxicated after 2 days (10 of 12 survivors; P = 0.0001) and 3 days (6 of 10 survivors; P = 0.011). Although pretreatment with B8C1ad did not increase survival rates in mice intoxicated 4 days later (2 of 10 survivors; P = 0.47 versus vehicle), B8C1ad pretreatment did prolong median survival time from 1 to 4 days (P = 0.003; Fig. 3D).
B8C1ad has an expanded therapeutic window compared to antitoxin
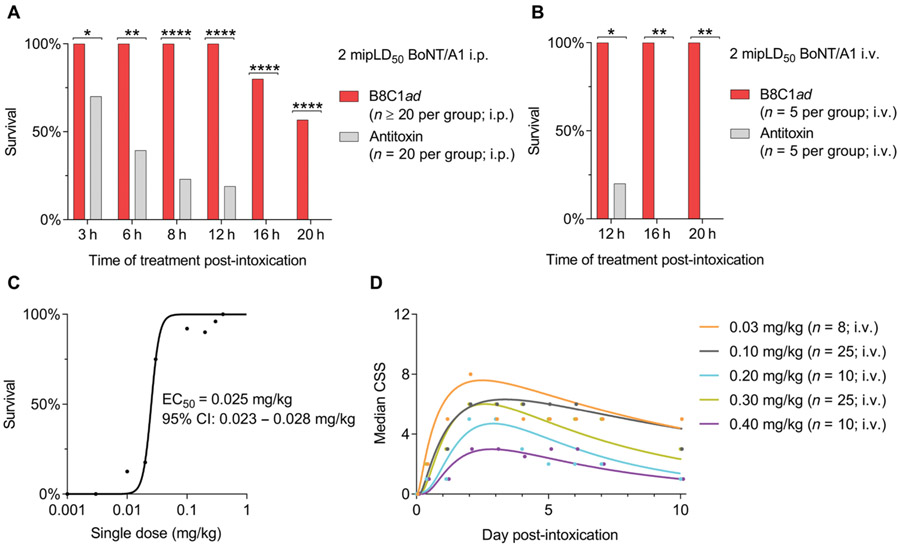
Antitoxin has a narrow therapeutic window such that the majority of botulism patients given antitoxin after symptomatic emergence require intensive care unit (ICU) support and artificial ventilation for survival (13, 14). Given the intracellular mechanism of action of B8C1ad, we hypothesized that B8C1ad would have a broader treatment window than antitoxin. To test this hypothesis, mice were challenged with 2 mipLD50 BoNT/A1 intraperitoneally and treated 3 to 20 hours later by intraperitoneal administration of B8C1ad or sufficient anti-BoNT/A1 serum to neutralize 10,000 mipLD50 of BoNT/A1 (21). Treatment with B8C1ad improved survival rates versus antitoxin at all post-exposure time points (Fig. 4A and table S3). To confirm that intraperitoneal administration did not reduce antitoxin efficacy, this study was repeated using intravenous administration of antitoxin or B8C1ad at 12, 16, and 20 hours after challenge with 2 mipLD50 BoNT/A1 intraperitoneally (n = 5 per group; table S3). Intravenous B8C1ad administration improved survival versus antitoxin at treatment latencies of 12 hours (antitoxin: 1 of 5 versus B8C1ad: 5 of 5, P = 0.048), 16 hours (antitoxin: 0 of 5 versus B8C1ad: 5 of 5, P = 0.008), and 20 hours (antitoxin: 1 of 5 versus B8C1ad: 5 of 5, P = 0.008; Fig. 4B), indicating that B8C1ad has a broader treatment window than antitoxin.
Fig. 4. B8C1ad has a longer therapeutic window than polyclonal antitoxin sera.
(A) Ten-day survival rates in mice challenged with BoNT/A1 (2 mipLD50, intraperitoneally) and treated with B8C1ad (0.4 mg/kg, intraperitoneally) or antitoxin (1 U, intraperitoneally) after 3 hours (n = 20), 6 hours (n = 20), 8 hours (n = 20), 12 hours (n = 30), 16 hours (n = 20), or 20 hours (n = 30). (B) Ten-day survival rates in mice challenged with BoNT/A1 (2 mipLD50, intraperitoneally) and treated with B8C1ad [0.3 mg/kg; intravenously (i.v.)] or antitoxin (1 U, intravenously) after 12 hours (n = 5), 16 hours (n = 5), or 20 hours (n = 5). (C) B8C1ad ED50 values were determined in mice challenged with BoNT/A1 (2 mipLD50, intraperitoneally) and treated with B8C1ad (0.003 to 0.40 mg/kg, intravenously) after 10 hours (n = 8 to 23 per dose). (D) Median clinical severity score (CSS) of groups in (C) with greater than 50% survival. (A), (C), and (D) represent the aggregate of at least three experiments; (B) represents the aggregate of two experiments. Additional details of statistical comparisons are presented in table S3. *P < 0.5, **P < 0.01, and ****P < 0.0001.
To determine whether B8C1ad exhibited dose-dependent protective effects on survival, mice (n = 8 to 23 per dose) were intoxicated with 2 mipLD50 BoNT/A1 and treated 10 hours later by intravenous administration of vehicle or B8C1ad (0.003 to 0.40 mg/kg). B8C1ad had monotonic effects on survival (R2 = 0.98), with a median effective concentration (EC50) of 0.025 mg/kg (95% confidence interval, 0.023 to 0.028; Fig. 4C). Toxic signs of botulism manifested within 1 day, peaked at 2 days, and were partially resolved by 10 days (Fig. 4D). Whereas B8C1ad doses of 0.003 mg/kg (0 of 10 survivors; P > 0.99), 0.01 mg/kg (1 of 8 survivors; P = 0.95), and 0.02 mg/kg (3 of 17 survivors; P = 0.15) were ineffective compared to vehicle (0 of 23 survivors), treatment with B8C1ad (0.03 to 0.4 mg/kg) improved survival rates to 75 to 100% (P < 0.0001 at each dose versus vehicle; table S3). Similarly, median toxic signs at 10 days were improved by treatment with B8C1ad (0.1 to 0.4 mg/kg) (P < 0.0001 at each dose versus vehicle; table S3). In addition to demonstrating a dose-response effect of B8C1ad in treatment of botulism, these data confirmed that NOAEL doses of B8C1ad did not elicit cumulative toxicities in BoNT-intoxicated mice.
B8C1ad antidotal rescue of guinea pig following lethal BoNT/A1 challenge
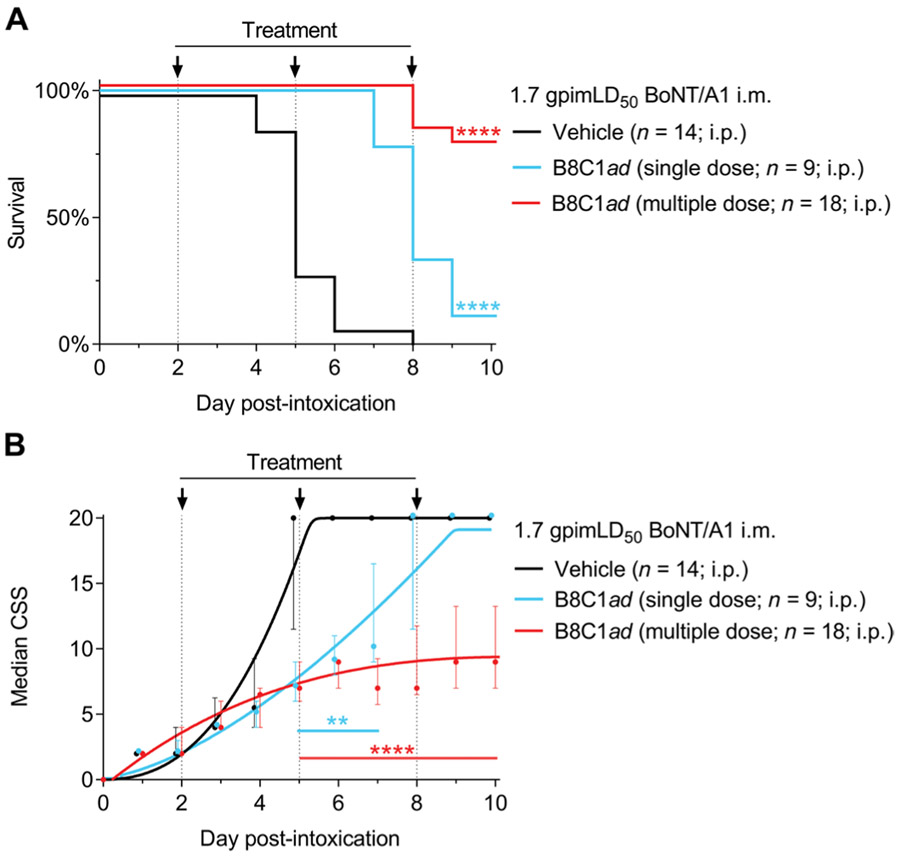
We next evaluated therapeutic efficacy of B8C1ad in BoNT/A1-intoxicated Hartley guinea pigs. Guinea pigs were previously used to support HBAT approval under the U.S. Food and Drug Administration animal efficacy rule, and thus, the pathogenesis of botulism and toxicity of BoNT/A1 in guinea pigs are well characterized (22). Unlike most rodents, guinea pigs exhibit a slow progression of botulism signs with a delayed onset of respiratory failure (23). To produce a delayed systemic intoxication model that resembles oral BoNT exposure, guinea pigs were challenged by injection of BoNT/A1 (19 mipLD50/kg) into the right hind limb gastrocnemius muscle (22). In pilot toxicity studies, this dose was determined to be 1.7 guinea pig intramuscular LD50 (1.7 gpimLD50). In control studies, weakness of the injected limb became apparent after 1 day, respiratory toxic signs emerged at 2 days, and guinea pigs expired around 5 days. Consistent with a post-exposure treatment paradigm, guinea pigs were given vehicle or B8C1ad (0.03 mg/kg, the guinea pig NOAEL; table S1) at 2 days after intoxication. Although intravenous treatment was presumed to offer more rapid distribution of B8C1ad, vascular access was limited by dehydration in intoxicated guinea pigs, and thus, treatments were given intraperitoneally. One group of guinea pigs was given a single dose of B8C1ad at 2 days, and a second group was given supplemental quarter doses of B8C1ad at 2, 5, and 8 days. For consistency, surviving guinea pigs in the vehicle treatment group were given supplemental vehicle injections at 5 and 8 days.
Vehicle-treated guinea pigs exhibited 0% (0 of 14) survival with a median survival time of 5 days (range, 4 to 8 days). Single-dose B8C1ad treatment had partial therapeutic efficacy, prolonging median survival time to 8 days (P < 0.0001 versus vehicle; Fig. 5A) but not improving the 10-day survival rate (1 of 9 survivors; P = 0.39 versus vehicle). In comparison, multiple-dose treatment with B8C1ad improved survival rates to 77.8% (14 of 18 survivors; P < 0.0001 versus vehicle). Consistent with improved survival in the multiple-dose treatment group, toxic signs of botulism deviated from vehicle-treated guinea pigs starting at 5 days and stabilized from 6 to 10 days after challenge (Fig. 5B). Thus, B8C1ad provided antidotal rescue in a symptomatic treatment model in a second species, with multiple treatments having improved therapeutic benefit compared to single treatment.
Fig. 5. B8C1ad antidotal rescue in guinea pigs lethally challenged with BoNT/A1.
Guinea pigs were challenged with 24 mipLD50 BoNT/A1 [1.7 guinea pig intramuscular (i.m.) LD50 (gpimLD50)] and treated 2 days later with vehicle (intraperitoneally; n = 14), a single dose of B8C1ad (0.02 mg/kg, intraperitoneally; n = 9), or multiple doses of B8C1ad (0.02 mg/kg) followed by supplemental treatments of B8C1ad at 5 and 8 days after intoxication (0.02 mg/kg, intraperitoneally; n = 18). Survival and CSS were evaluated at daily intervals. (A) Ten-day survival curves comparing vehicle, single-dose B8C1ad, and multiple-dose B8C1ad. (B) Median ± interquartile ratio (IQR) CSS scores. All data represent the aggregate of at least three experiments. Additional details of statistical comparisons are presented in table S3. **P < 0.01 and ****P < 0.0001.
B8C1ad antidotal rescue of rhesus macaque after lethal BoNT/A1 challenge
We next evaluated the effects of intravenous B8C1ad treatment on 10-day survival in Chinese-origin rhesus macaques. The pathogenesis of botulism is clinically similar between humans and rhesus macaques (24, 25), and thus, rhesus macaques offered a highly translatable model for therapeutic testing. In pilot studies, rhesus macaques challenged with BoNT/A1 (40 mipLD50/kg) [1.7 rhesus macaque intravenous LD50 (1.7 rmivLD50)] (20) exhibited acute signs of botulism within 1 day and expired by 3 days. In efficacy studies, intoxicated monkeys were given intravenous injections of vehicle (n = 7) or B8C1ad (0.06 mg/kg) (n = 6; the rhesus macaque NOAEL; table S1) at 1 day after intoxication (Fig. 6A). Monkeys exhibited either mild ptosis or mild abdominal respiration at time of treatment (Fig. 6B). On the basis of guinea pig findings that multidose treatment provided therapeutic benefit, B8C1ad-treated monkeys were given additional quarter doses of B8C1ad at 4 and 7 days. The median survival time of vehicle-treated monkeys was 3.0 days (range, 2.75 to 3.5 days) after BoNT/A1 challenge, with no monkeys surviving beyond 3.5 days (Fig. 6A). In comparison, 100% of B8C1ad-treated monkeys survived to 10 days (P = 0.0006 versus vehicle). Toxic signs deviated between the treatment groups starting at 2.5 days and stabilized in the B8C1ad-treated group from 4.5 to 10 days (Fig. 6B). The majority of B8C1ad-treated monkeys exhibited partial reversal of dysphagia, abdominal paradox, and ptosis by 8 days, and all monkeys resumed consumption of liquids and food by 9 days. These data demonstrated therapeutic success of B8C1ad in a highly relevant preclinical model of botulism.
Fig. 6. B8C1ad antidotal rescue in rhesus macaques lethally challenged with BoNT/A1.
Rhesus macaque monkeys were challenged with 40 mipLD50 BoNT/A1 [1.7 rhesus macaque intravenous LD50 (rmivLD50), intravenously] and treated by intravenous administration of B8C1ad (0.06 mg/kg) (green, n = 6) or vehicle (black, n = 7) at 24 hours after challenge. Surviving monkeys received additional treatments of B8C1ad (0.015 mg/kg) at 4 and 7 days after challenge and supplemental hydration twice daily starting at 4 days. Survival and CSS were evaluated at 6-hour intervals. (A) Ten-day survival curves. (B) Median ± IQR CSS scores. All data represent the aggregate of at least three experiments. Additional details of statistical comparisons are presented in table S3. ***P < 0.001.
DISCUSSION
Here, we report that an atoxic BoNT derivative can be used to deliver therapeutic antibodies to the neuronal cytosol, where they protect SNAP-25 from BoNT/A1 metalloprotease activity. In proof-of-concept studies in mice, we demonstrate that post-symptomatic delivery of an LC/A1 function-blocking antibody halts botulism progression, reverses paralysis, and promotes survival after lethal challenge with BoNT/A1. We further demonstrate antidotal rescue in guinea pigs and rhesus macaque monkeys, which have been used as clinical surrogates for approval of botulism countermeasures (20, 23). By repurposing an atoxic version of BoNT/C1 as a molecular delivery vehicle, we demonstrate the ability to deliver therapeutic protein moieties to neurons with high precision, producing the first therapeutic to exhibit in vivo efficacy in experimental models of botulism. These findings are corroborated by another manuscript in this issue, which demonstrates treatment of botulism in mice challenged with BoNT/A or BoNT/B using therapeutic antibodies fused to an atoxic derivative of BoNT/XA (26).
Previous reports have purported to use BoNT derivatives or subunits to deliver payloads to the cytoplasmic compartment of neurons (27-31). In most cases, the payload remains associated with endosomal or lysosomal compartments, without convincing evidence of cytoplasmic delivery (28-31). In an alternative approach, the neuronal specificity of BoNT/E has been harnessed to explore the therapeutic effects of multiple serotypes on recovery after BoNT/A1 intoxication (32). Here, we provide extensive biochemical, colocalization, and functional evidence in multiple animal models to demonstrate that atoxic BoNT/C1 delivers therapeutic proteins to the intraneuronal space of intoxicated motor neurons. The success of this proof-of-concept delivery vehicle suggests that scaffolds derived from other BoNT and BoNT-like toxins could be used to increase therapeutic range, target distinct neuronal populations, or modify intracellular properties of therapeutic cargo, such as persistence and trafficking.
B8C1ad exhibited variable species-specific dose-dependent toxicities, with NOAEL doses encompassing a 10-fold range among mice, guinea pigs, and monkeys. Similar species-specific susceptibilities to BoNTs are well described (23, 33), although in most cases the underlying mechanism(s) is unknown. NOAEL differences could result from changes in pharmacokinetic parameters, receptor binding affinities and/or B8C1ad uptake, trafficking, or proteolytic activity in nerve terminals. Although the etiology of B8C1ad toxicity remains unclear, B8LC retains the ability to interact with target SNARE proteins and consequently may interfere with the formation of ternary SNARE complexes at release sites. B8C1ad offered strong therapeutic benefit in all species at NOAEL doses, suggesting that toxicity is proportional to therapeutic efficacy.
The flexibility of the C1ad molecular delivery platform offers several advantages for the rapid generation of new treatments for neurological disorders. In particular, the presynaptic localization of LC suggests that this therapeutic approach will be particularly effective in treating synaptopathies involving active zone proteins (34). The platform can be efficiently redirected toward other protein targets by replacing or adding sdAbs or other protein moieties (6, 17, 35). For example, BoNT serotypes A to F include 33 BoNT subtypes. Within each serotype, LC active sites exhibit a high degree of primary sequence identity (6). It is, therefore, feasible that the tandem addition of one to two sdAbs to C1ad will provide broad-spectrum inhibition of subtype LCs from a single BoNT serotype (36). In combination with robust methods for engineering and selection of sdAbs against relevant epitopes, the modularity of the C1ad delivery platform enables it to be repurposed for the rapid generation of broad-spectrum therapeutics.
Several options are available to improve the general utility of B8C1ad. First, sdAb binding affinity for LC/A1 can be increased via incorporation of tandem sdAbs that simultaneously bind different epitopes (37). Second, B8C1ad specificity can be expanded by incorporating sdAbs that target the LC proteases of other BoNT serotypes. Potency can also be increased by addition of a modular ubiquitin ligase substrate recognition domain to target the sdAb-bound toxin LCs for proteasomal degradation. Reducing the therapeutic dose will both increase the therapeutic index and decrease the likelihood of eliciting an immune response. Similar constructs have been successfully tested with BoNT/A1 in cell assays (38) and more broadly in eukaryotes (39).
Weaponized BoNT remains a tier 1 bioterror threat, largely because of the lack of effective countermeasures. Although antitoxin offers prophylactic benefit if administered before symptomatic onset, the majority of symptomatic botulism patients treated with HBAT still require ICU support (>88%) and artificial ventilation (>60%) for survival (13, 14). A monoclonal antitoxin cocktail against BoNT serotypes A and B with improved safety profile and serum half-life compared to HBAT is in phase 2 clinical trials (40), although it is not anticipated to improve the post-exposure window for therapeutic prophylaxis. The recent discovery of small molecules that interfere with intercellular translocation may extend the prophylactic window provided by antitoxin treatment (41). Recent reports suggest that 3,4-diaminopyridine (3,4-DAP) may offer yet a third mechanism to temporarily reverse toxic signs of botulism (42). These ongoing studies offer the prospect of a multimodal approach to comprehensive treatment of symptomatic botulism, using a combination of antitoxin to neutralize toxin in blood, 3,4-DAP to temporarily reverse respiratory paralysis, and an antidote to terminal intracellular toxicity. Our data suggest that B8C1ad will contribute to a potent multimodal therapy by inactivating BoNT/A1 LC protease within neurons, and thereby reversing the lethal BoNT/A1 effects on neurotransmission.
In vivo studies indicate that early administration of B8C1ad (or a similar drug) to botulism patients intoxicated by BoNT/A1 will reduce the need for intubation and/or accelerate extubation, thus mitigating life-threatening side effects and costs associated with prolonged intensive care (43, 44). However, it is unknown whether the long-term paralysis caused by lethal doses of BoNT/A1 results from persistent LC activity (45) or prolonged residence of cleaved SNARE proteins in the nerve terminal (32). Furthermore, the relative persistence of LC/A1 and B8LC within the nerve terminal is unclear. Consequently, although B8C1ad is therapeutically effective during acute stages of botulism, the duration of the therapeutic window and the number of treatments required to promote recovery from paralysis remain to be determined.
BoNT/C1 is not a natural human poison, and there are no clinical data on the elicitation of immune responses after BoNT/C1 exposure. Immunoglobulin G (IgG) immune responses were elicited by bolus administration of a related BoNT-derived delivery vehicle at a 10-fold lower dose, suggesting that treatments with B8C1ad may also be immunogenic (46). However, botulism is a rare disease, and it is unlikely that one person will require treatment for multiple cases of botulism (47). Consequently, we anticipate that B8C1ad will be administered as a single-use treatment or as multiple-dose treatment over a short time frame, mitigating concern about immunogenic responses.
Although B8C1ad showed strong therapeutic benefits in three experimental models of botulism, our interpretation of the underlying mechanism is subject to technical limitations. First, whereas we report convincing data that B8LC traffics to the presynaptic compartment and engages with LC/A, we do not provide direct evidence of cytosolic localization of B8LC. We continue to pursue this question using fusion protein-based labeling techniques with electron microscopy approaches. Second, our data suggest that B8LC inhibits LC/A1 activity until LC/A1 is cleared from the nerve terminal through natural processes. However, we did not demonstrate full recovery of intact SNAP-25 in vitro. Thus, we have not yet determined the intracellular half-lives of B8LC compared to LC/A1 nor whether the therapeutic mechanism of action is restricted to inhibition of LC/A1 proteolytic activity or includes facilitation of LC/A1 clearance. Third, the C1ad molecular vehicle exhibits acute toxicity at supratherapeutic doses in each animal model studied. It will be important to determine a clinically safe posology and to develop therapeutic moieties with improved therapeutic indices. Last, our data suggest that therapeutic moieties fused to the C1ad LC may specifically traffic to the presynaptic compartment. Consequently, indications involving therapeutic targets in other neuronal compartments may require trafficking signals to redirect the LC accordingly.
In summary, we demonstrated in vivo delivery of therapeutic proteins to the cytoplasmic compartment of neurons without requiring transfection or viral vectors. Using clinical botulism as a proof-of-concept therapeutic target, we demonstrate that post-symptomatic administration of B8C1ad reverses botulism signs and enhances survival in three species after a lethal BoNT/A1 challenge. Consistent with an intraneuronal mode of action, post-intoxication treatment with B8C1ad provides benefit at times when passive immunization with antitoxin is wholly ineffective. This platform offers a transformational approach for a precision treatment that might be adapted to diverse presynaptic pathologies through delivery of functional antibodies or other protein moieties to previously inaccessible intraneuronal targets.
MATERIALS AND METHODS
Study design
The main objective of the study was to develop and test a post-exposure botulism antidote when administered to symptomatic animals after lethal botulism challenge. After in vitro validation of therapeutic mechanisms, efficacy studies were conducted in mice, guinea pigs, and rhesus macaque monkeys. Before efficacy testing in each species, NOAEL doses of B8C1ad were determined by administration of escalating doses until adverse effects were observed.
For efficacy studies, sample sizes were determined by power analysis in StatMate 2 (GraphPad Software) using conservative effect sizes. All mouse efficacy studies involved three or more separate experiments, with each experiment including all treatment groups, with the exception of Fig. 4B, which was repeated twice. Guinea pig and monkey efficacy studies were conducted in small groups (maximum of six guinea pigs or three monkeys per experiment), with each experiment including all treatment groups. Mouse and guinea pig safety studies were repeated in at least three animals per B8C1ad dose, whereas monkey safety studies involved a single animal at each B8C1ad dose. For determination of LD50 and ED50 values (Fig. 3A), an adaptive, stagewise approach over at least five trials was taken, in which early trials used a wide dose range that was narrowed in subsequent trials.
Mice, guinea pigs, and monkeys were randomized before intoxication, and experimenters were unblinded to the groups only after treatments were administered. The number of replicates, statistical tests, and outcomes for each experiment are summarized in the text and detailed in table S3. No outliers were excluded during data analysis. Only animals identified by a veterinarian to be in poor health for reasons unrelated to the study protocol were excluded from data analysis. We excluded three monkeys (one vehicle and two treatment group) that developed a severe fever after intoxication but before treatment with vehicle or B8C1ad. We excluded two mice that were given toxin but failed to develop symptoms of botulism before B8C1ad treatment at 24 hours.
Animal studies were conducted with the approval of Institutional Animal Care and Use Committees at New York University Grossman School of Medicine (NYU GSoM), City University of New York, City College (CCNY), Cummings School of Veterinary Medicine at Tufts University (CSVMTU), Mispro/Alexandria Center for Life Sciences (CytoDel Inc.), and United States Army Medical Research Institute of Chemical Defense (USAMRICD). NYU GSoM, USAMRICD, CCNY, Mispro/CytoDel Inc., and CSVMTU have approved Animal Welfare Assurance Agreements (A3435-01, A4528-01, A3733-01, D16-00884, and A4059-01, respectively) on file with the National Institutes of Health (NIH) Office of Laboratory Animal Welfare. All procedures were conducted in accordance with the principles stated in the Guide for the Care and Use of Laboratory Animals (48) and the Animal Welfare Act of 1966 (P.L. 89-544), as amended. Nonhuman primate studies were conducted with additional review and approval by the Animal Care and Use Review Office in the U.S. Army Medical Research and Development Command’s Office of Research Protections. All animals intoxicated with BoNT or treated with B8C1ad were monitored at least twice daily for euthanasia criteria, which included morbidity exceeding 24 hours in duration, weight loss exceeding 30%, or emergence of agonal breathing. The Institutional Review Entity at NYU GSoM has reviewed and determined that the work does not fall under Dual Use Research of Concern.
Reagents/supplies
Reagents included ammonium bicarbonate (Acros Organics, catalog no. 393212500), d-desthiobiotin (MilliporeSigma, catalog no. D1411), dl-dithiothreitol (DTT) (MilliporeSigma, catalog no. D0632-5G), formaldehyde, 16% solution (Electron Microscopy Sciences, catalog no. 15710), formic acid (Thermo Fisher Scientific, catalog no. A117-50), l-glutathione oxidized (MilliporeSigma, catalog no. G4376), l-glutathione reduced (MilliporeSigma, catalog no. G4251), imidazole (Thermo Fisher Scientific, catalog no. BP305-50), β-mercaptoethanol (Bio-Rad, catalog no. 1610710), polyethylenimine (MilliporeSigma, catalog no. 408727), polyethylenimine/laminin-coated glass coverslips (MilliporeSigma), phosphate-buffered saline (PBS)–G (MilliporeSigma, catalog no. G0411), 10% Triton X-100 (Surfact-Amps X-100, Thermo Fisher Scientific, catalog no. 28314), 1× Dulbecco’s PBS (Thermo Fisher Scientific, catalog no. 21-030-CV), 2% sterile gelatin solution (MilliporeSigma, catalog no. G1393), Criterion XT 4–12% Bis-Tris gel (Bio-Rad, catalog no. 3450124), XT MES Running Buffer (Bio-Rad, catalog no. 1610789), 4× Laemmli sample buffer (Bio-Rad, catalog no. 1610747), Coomassie Blue R-250 stain solution (Teknova, catalog no. C1050), 4–12% Bis-Tris NuPAGE gel (Thermo Fisher Scientific, catalog no. NP0323BOX), 20× NuPAGE MOPS SDS running buffer (Thermo Fisher Scientific, catalog no. NP000102), 4× NuPAGE LDS sample buffer (Thermo Fisher Scientific, catalog no. NP0007), BCA assay kit (Thermo Fisher Scientific, catalog no. 23227), nitrocellulose membranes (Bio-Rad, catalog no. 162–0168), thick blot paper (Bio-Rad, catalog no. 1703956), normal goat serum (Thermo Fisher Scientific, catalog no. 16210–072), SuperSignal West Pico chemiluminescent substrate (Thermo Fisher Scientific, catalog no. 34080), BL21 (DE3) CodonPlus-RIL cells (Agilent Technologies, catalog no. 230245), pRK793 6-His-TEV protease plasmid (Addgene, catalog no. 8827), saponin (MilliporeSigm, catalog no. 558255), Neurobasal Medium (Thermo Fisher Scientific, catalog no. 21103049), B27 Supplement (Thermo Fisher Scientific, catalog no. 17504044), NbActiv4 medium (BrainBits LLC, catalog no. Nb4-500), E18 rat cortical tissue (BrainBits LLC, catalog no. SDECX), SF-900 II serum-free medium (Thermo Fisher Scientific, catalog no. 10902088), NeuroCult SM1 Supplement (STEMCELL Technologies, catalog no. 05711), anti–neurofilament-M (NFIL; MilliporeSigma, catalog no. MA1621), IgG-free bovine serum albumin (BSA) (MilliporeSigma, catalog no. A0336), glass coverslips coated with poly-d-lysine (MilliporeSigma, catalog no. P6407), anti–β-actin mouse IgG2a (MilliporeSigma, catalog no. A5316), anti–EEA-1 mouse IgG (BD Biosciences, catalog no. 610457), anti–hemagglutinin (HA) 3F10 rat IgG (MilliporeSigma, catalog no. 11867423001), anti–lysosomal-associated membrane protein–1 (LAMP-1) rabbit IgG (MilliporeSigma, catalog no. AB2971), anti–Rab-7 mouse monoclonal antibody (mAb) (Santa Cruz Biotechnology, catalog no. sc-376362), anti–mitogen-activated protein 2 (MAP2) chicken IgY (Abcam, catalog no. ab5392), anti–SNAP-25 mouse IgG (Synaptic Systems, catalog no.111011), anti-STX1 mouse IgG (Synaptic Systems, catalog no. 106011), anti-rat Tau mouse IgG2b (BD Biosciences, catalog no. 610672), Alexa Fluor 555 goat anti-human IgG (Thermo Fisher Scientific, catalog no. A-21433), Alexa Fluor 555 goat anti-mouse IgG2b (Thermo Fisher Scientific, catalog no. A-21422), Alexa Fluor 647 goat anti-mouse IgG2b (Thermo Fisher Scientific, catalog no. A-21242), Alexa Fluor 647 goat anti-rabbit IgG (Thermo Fisher Scientific, catalog no. A-21245), Alexa Fluor 488 goat anti-human IgG (Thermo Fisher Scientific, catalog no. A-11013), horseradish peroxidase (HRP) anti–E-tag mAb (GE Healthcare), 3,3′,5,5′-tetramethylbenzidine (MilliporeSigma, catalog no. 860336), anti-OLLAS tag (rat mAb; Thermo Fisher Scientific, catalog no. MA5-16125), c-myc tag (mouse mAb; Thermo Fisher Scientific, catalog no. 13-2500), E-tag [rabbit polyclonal antibody (pAb); Abcam, catalog no. ab3397], anti-BoNT/C1 LC [human mAb 4C10.2; J. Marks, University of California San Francisco (UCSF)], anti-BoNT/C1 HC (human mAb 8DC1.2; J. Marks, UCSF), anti-HA tag (mouse mAb 3F10; MilliporeSigma, catalog no. 11867423001), rabbit anti-BoNT/A1 (pAb Pol001; Statens Serum Institut, Denmark), BoTest A/E BoNT Detection Kit (BioSentinel Inc., catalog no. A1004), sheep pAb against BoNT/C1 receptor-binding domain (R&D Systems, catalog no. AF5425), ProLong Gold 4′,6-diamidino-2-phenylindole (DAPI) mounting media (Thermo Fisher Scientific, catalog no. P36935), Ni2+-NTA Sepharose fast flow (IBA GmbH, catalog no. 2-3206-025), StrepTactin Sepharose fast flow (IBA GmbH, catalog no. 2-1208-025), HiLoad 26/600 Superdex 200 pg gel filtration column (GE Healthcare Life Sciences, catalog no. 28989336), and 0.22-μm filters (MilliporeSigma, catalog no. UFC30GV0S). All other chemicals were purchased from either Thermo Fisher Scientific or MilliporeSigma, unless indicated otherwise.
Animals
CD-1 female mice (22 to 30 g) (Charles River Laboratories) were housed five per cage in a barrier facility. Male guinea pigs (400 to 500 g; Charles River Laboratories) were individually housed in a barrier facility. Chinese-origin rhesus macaques (5 to 6 years of age, mixed gender, 8 to 13 kg) were individually housed in a group housing facility. All animals were maintained on a 12-hour light/dark cycle with ad libitum access to food and water. For each lot of BoNT/A1, the specific activity was determined in vivo via nonlinear regression analysis using the mouse intraperitoneal lethality assay (49).
Analysis of BoNT/A1 metalloprotease activity inhibition by B8C1ad in vitro
BoNT/A1 (100 pM) was combined with 1 to 81 molar equivalents of B8C1ad or C1ad in the presence of 10 mM DTT. The mixtures were kept for 10 min at 25°C and then added to the BoTest FRET reporter substrate (BioSentinel Inc.). The reaction was incubated at 37°C for 24 hours. Aliquots from each reaction were loaded in duplicate on a 96-well black plate, and absorbance at 526 and 470 nm was measured on a spectrophotometer. Positive controls were FRET substrate without any additional protein components (reporter only), and FRET substrate plus 8.1 nM B8C1ad. The negative control was FRET substrate incubated with 100 nM BoNT/A1.
Analysis of primary neuronal cultures treated with BoNT/A1 and chased with B8C1ad
E18 rat cortical tissue (BrainBits LLC) was plated at a density of 125,000 cells/cm2 on plasma-cleaned, polyethylenimine/laminin-coated glass coverslips as previously described (50). Neuronal cultures were maintained at 5% CO2, 37°C, and 95% humidity in NbActiv4 medium. Experiments were performed 18 to 20 days after plating. For neuronal treatments, BoNT/A1 and B8C1ad were separately prepared at 100× final concentration in fresh NbActiv4 medium. Neurons were exposed to 670 pM BoNT/A1 for 2 hours and chased for 2 hours with fresh medium followed by incubation with 10 nM B8C1ad for 16 hours. Neurons were briefly washed with PBS, fixed for 15 min in ice-cold 4% formaldehyde, and blocked with 3% BSA, 0.1% saponin in PBS as previously described (51). Neurons were stained for nuclei (DAPI), NFIL (1:250), OLLAS (tag on the N terminus of B8LC, 1:75), and LC/A1 (1:2000, mAb 5A20.4; gift of J. Marks, UCSF) and detected using Alexa Fluor 647–labeled goat anti-mouse, Alexa Fluor 488–labeled goat anti-rat, and Alexa Fluor 555–labeled goat anti-human secondary antibodies (each at 1:500). All antibody dilutions were made in blocking solution. Stained coverslips were mounted with ProLong Gold antifade mounting medium, and epifluorescence images were captured with standard excitation and emission filters using a Zeiss 700 confocal microscope.
Reciprocal coimmunoprecipitation studies
E18 rat cortical neurons (BrainBits LLC) were plated at 5.4 × 104 cells/cm2 in six-well dishes and maintained for 14 days in Neurobasal medium supplemented with B27. Neurons were incubated with 6 nM BoNT/A1 for 24 hours, washed with 50% conditioned medium, chased for 2 hours with 50% conditioned medium, incubated with 50 nM B8C1ad for 24 hours, and chased with 50% conditioned medium for another 24 hours. Neurons were lysed on ice in Triton X-100 lysis buffer supplemented with protease inhibitors. Lysates were clarified by high-speed centrifugation, and protein concentration in lysates was normalized. For coimmunoprecipitation studies, lysates were incubated with human anti-LC/A1 mAb 5A20.4 (J. Marks, UCSF) or rat anti-OLLAS tag (for capture of B8LC), followed by incubation with protein G Sepharose beads. Beads were pelleted and incubated in 2× SDS–polyacrylamide gel electrophoresis (PAGE) loading buffer with β-mercaptoethanol, and 30 μg of input and elution sample was separated on 4 to 12% Criterion SDS-PAGE gels and transferred to 0.2-μm nitrocellulose membranes. Membranes were incubated with antibodies against OLLAS tag or BoNT/A1 overnight at 4°C, followed by incubation with HRP-conjugated secondary antibodies for 45 min at room temperature. SuperSignal West Pico chemiluminescent substrate was used for visualization. Western blot images were acquired using a LiCor Odyssey Fc imaging system and analyzed by densitometry using instrument software. Coimmunoprecipitation studies were replicated four times with similar results.
Examination of stability and persistence of B8LC after internalization of B8C1ad in cortical neurons
Rat E18 cortical neurons (BrainBits LLC) were plated at 62,000 cells/cm2 and maintained for 14 days. Neurons were exposed to 50 nM B8C1ad for 48 hours, washed, and incubated for an additional 3 hours or 1, 3, 5, or 7 days. Neurons were lysed in 0.5% Triton X-100 with protease inhibitors, and 50 μg of total protein was separated by SDS-PAGE. For immunoblots, membranes were blocked in 10% fat-free milk supplemented with 5% normal goat serum in TBST [150 mM NaCl, 10 mM tris-HCl (pH 8.0), and 0.1% Tween 20] at room temperature for 1 hour. Membranes were incubated with primary antibodies overnight at 4°C and with secondary antibodies for 30 min at room temperature. Anti–E-tag was used to detect B8C1ad, and anti–β-actin mAb was used as a loading control. After incubations, blots were washed with TBST three times for 5 min. SuperSignal West Pico chemiluminescent substrate was used for visualization. Western blot images were acquired using a LiCor Odyssey Fc imaging system. This experiment was replicated four times with similar results.
Determination of B8C1ad LD50 and NOAEL in CD-1 mice
CD-1 mice were injected intraperitoneally with 0.5 ml of vehicle or B8C1ad (1 to 2.4 mg/kg) prepared in saline with 0.2% gelatin. Survival was monitored daily for 10 days. Female CD-1 mice received intraperitoneal injections of B8C1ad (0.2, 0.4, 0.6, or 0.8 mg/kg) in saline with 0.2% gelatin. Toxic signs were monitored daily for 10 days using the following clinical severity score (CSS) rubric modified from (42): (A) respiratory signs: mild abdominal paradox (score of 1), moderate abdominal paradox (3), or severe abdominal paradox and/or agonal respiratory pattern (6), plus (B) skeletomuscular signs: salivation (1), lethargy (1), limb weakness (3), and total body paralysis (lack of righting reflex, 6). Animals were given a score of 16 upon death or euthanasia.
B8C1ad efficacy in mice
For each lot of BoNT/A1, the specific activity was determined via nonlinear regression analysis using the mouse intraperitoneal lethality assay (49). CD-1 mice were intoxicated with BoNT/A1 (2 mipLD50; intraperitoneally). Vehicle (saline with 0.2% gelatin), B8C1ad (0.4 mg/kg), sdAb B8 protein (0.04 mg/kg), or C1ad (0.35 mg/kg) was injected intraperitoneally at 12 hours after BoNT/A1 challenge. Subcutaneous hydration with 1.0-ml saline was administered at 12-hour intervals to all surviving mice on days 2, 3, 4, and 5. For 4 and 6 LD50 studies, CD-1 mice were challenged with 4 or 6 mipLD50 of BoNT/A1 (intraperitoneally). Animals were treated with vehicle (intraperitoneally) or B8C1ad (0.4 mg/kg, intraperitoneally) at 5.5 hours after intoxication. Subcutaneous hydration with 0.5-ml saline was administered at 12-hour intervals from 2 to 6 days.
Prophylactic properties of B8C1ad tested in mice
Mice were pretreated with B8C1ad (0.4 mg/kg; intraperitoneally) and challenged with BoNT/A1 (2 mipLD50; intraperitoneally) at 2, 3, or 4 days later. As a negative control, mice were treated with saline with 0.2% gelatin at 2 days before challenge. BoNT/A1 and B8C1ad were prepared in saline with 0.2% gelatin. Subcutaneous hydration with 0.5-ml saline was administered at 12-hour intervals from 2 to 6 days.
Efficacy of B8C1ad compared to antitoxin treatment
To evaluate the post-exposure efficacy of B8C1ad, mice were challenged with BoNT/A1 (2 mipLD50; intraperitoneally) and administered B8C1ad (0.4 mg/kg; intraperitoneally) or 1 U of antitoxin (enough to neutralize 10,000 mipLD50 BoNT/A1; intraperitoneally) after 3, 6, 8, 12, 16, or 20 hours. BoNT/A1 and B8C1ad were prepared in saline with 0.2% gelatin. In another cohort, mice were administered B8C1ad (0.4 mg/kg; intravenously) or antitoxin (1 U; intravenously) at 12, 16, or 20 hours after BoNT/A1 challenge. Subcutaneous hydration with 0.5-ml saline was administered at 12-hour intervals from 2 to 6 days. Mice were evaluated for survival and CSS at 24-hour intervals.
Determination of intramuscular BoNT/A1 LD50 in guinea pigs
To establish BoNT/A1 potency in the guinea pig model, guinea pigs (400 to 500 g) were injected in the right hind limb gastrocnemius muscle with 8 to 15 mipLD50/kg (n = 3 per dose) and survival was monitored over 10 days. The guinea pig intramuscular LD50 was estimated by probit analysis to be 11 mipLD50/kg, consistent with previous report (22). In pilot and vehicle control studies, 0 of 34 guinea pigs survived intramuscular intoxication with BoNT/A1 (19 mipLD50/kg) (approximately 1.7 guinea pig intramuscular LD50).
Efficacy of B8C1ad in guinea pigs
To determine the NOAEL dose, guinea pigs were administered a 1-ml dose of B8C1ad (0.02, 0.03, 0.04, or 0.08 mg/kg) (intraperitoneally; table S1). Toxic signs were monitored daily over 10 days using the following CSS rubric modified from (22): lacrimation (score of 1); salivation (2); neuromuscular signs (select one): lethargy (1), injected limb weakness (2), generalized body weakness (3), or total paralysis (9); respiratory signs (select one): abdominal paradoxical breathing (2), forced abdominal breathing (5); deceased (20). For efficacy testing, guinea pigs were injected in the right hind limb gastrocnemius muscle with 19 mipLD50/kg (22). The vehicle-treated control group was given saline with 0.2% gelatin at 2 and 5 days after intoxication. The single-dose and multiple-dose treatment groups received B8C1ad (0.02 mg/kg; intraperitoneally) at 2 days after intoxication. The multiple-dose cohort received additional injections of B8C1ad (0.005 mg/kg) at 5 and 8 days after intoxication. BoNT/A1 and B8C1ad were prepared in saline with 0.2% gelatin. Guinea pigs were given 30 ml of subcutaneous hydration every 12 hours from 4 to 10 days after intoxication. Survival and CSS were evaluated daily.
Characterization of intravenous BoNT/A1 toxicity in rhesus macaque monkeys
To establish BoNT/A1 potency in rhesus macaque monkeys, macaques (6 to 10 kg) were exposed by intravenous injection to BoNT/A1 (40 or 44 mipLD50/kg). In pilot studies (n = 2 animals at each dose), macaques exposed to 40 mipLD50/kg died at 2.5 and 2.75 days, whereas macaques exposed to 44 mipLD50/kg died at 1.5 and 1.75 days. The natural course of disease was consistent with previous report (20), and thus, 40 mipLD50/kg was considered to be 1.7 rhesus macaque intravenous LD50 (1.7 rmivLD50). In pilot and vehicle control studies, no macaques survived beyond 3.5 days at this dose.
Efficacy of B8C1ad in rhesus macaque monkeys
To determine the NOAEL dose, rhesus macaque monkeys (5 to 6 years of age) were given bolus intravenous administration of B8C1ad with quarter-dose supplemental treatments at 3 and 6 days (table S1). Toxic signs were monitored every 6 hours for 10 days using the following CSS rubric modified from (20): ptosis: slight (score of 1), severe (2); muscle weakness: hunched (1), prostrate able to rise (2), prostate unable to rise (3); respiration: abdominal (1), abdominal with open mouth and grunting (2), agonal breathing (3); salivation: present (1); nasal discharge: present (1); deceased (16). Scores within each category were not cumulative. For efficacy studies, macaques were challenged with BoNT/A1 (40 U/kg; intravenously) and administered a 1-ml dose of vehicle (intravenous) or B8C1ad (0.06 mg/kg; intravenously) prepared in saline with 0.2% gelatin at 1 day after intoxication. Survival and clinical signs of botulism were evaluated every 6 hours for 10 days. Monkeys received supplemental treatments of B8C1ad (0.015 mg/kg) at 4 and 7 days after intoxication. Limited intravenous hydration with 50- to 100-ml warm lactated Ringer’s solution was given daily under mild ketamine anesthesia (2 mg/kg; intramuscularly) from 4 to 10 days after intoxication.
Statistical design
A detailed explanation of sample sizes, statistical methods, and significance thresholds for all comparisons is provided in table S3. Raw data were used for all analyses except for densitometric analyses of SNAP-25 cleavage in Fig. 2C and fig. S6A. The percentage of cleaved SNAP-25 in each lane was calculated by dividing the intensity of the cleaved band by the sum of the intensities of the cleaved and uncleaved bands and averaging among all samples in each condition. Threshold for significance was a = 0.05 unless adjusted for multiple hypothesis testing as described in table S3.
Supplementary Material
Fig. S1. sdAb B8 binds LC/A1 but not the intact BoNT/A1 heterodimer.
Fig. S2. B8C1ad expression, purification, and processing.
Fig. S3. Proteomic and immunological characterization of B8C1ad heterodimer.
Fig. S4. Internalized B8LC colocalizes with presynaptic markers in primary neurons.
Fig. S5. Internalized B8LC does not colocalize with endosomal, lysosomal, or autophagosomal markers in primary neurons.
Fig. S6. B8C1ad protects against BoNT/A1 challenge in primary neurons.
Table S1. Dose-dependent toxicities of B8C1ad in mice, guinea pigs, and rhesus macaques.
Table S2. Emergence of respiratory signs of botulism in mice challenged with 2 mipLD50 BoNT/A1.
Table S3. Sample sizes, statistical analyses, and significance thresholds for all comparisons.
Data file S1. Individual-level details for each experiment.
Data file S2. Individual CSS scores at all time points for guinea pig and rhesus macaque survival studies.
Reference (52)
Acknowledgments:
We thank E. A. Johnson and S. Pellett (University of Wisconsin at Madison) for earlier contributions to this work, J. Lou and J. Marks (University of California at San Francisco) for mAbs against BoNT/C1, Allergan Inc. for gift of research-grade BoNT/A1 used for nonhuman primate studies, and R. Webb (United States Army Medical Research Institute of Infectious Diseases) for recombinant, catalytically inactive (ci) BoNT/A1 protein. We appreciate the excellent technical assistance of J. Mukherjee, J. Tremblay, K. Baldwin, M. Debatis (Cummings School of Veterinary Medicine at Tufts University), and M. Stenslik (United States Army Medical Research Institute of Chemical Defense). We are also grateful to T. Südhof (Stanford University School of Medicine), R. Tsien, M. Chao, and E. Ziff (New York University Grossman School of Medicine), and H. Haimes and M. Johnson (Defense Threat Reduction Agency) for their ongoing discussion and critical reading of the manuscript. We also thank E. C. B. Milner for reading and careful editing of the manuscript. The views expressed in this manuscript are those of the authors and do not reflect the official policy of the Department of Army, Department of Defense, or the U.S. government.
Funding:
This work was conducted under funding from (i) NIH R01AI093504 to K.I., C.B.S., P.M.M., and P.A.B.; (ii) Defense Threat Reduction Agency (DTRA)–Joint Science and Technology Office, Medical S&T Division to P.M.M.; and (iii) CytoDel Inc. to P.M.M. This research was performed while J.B.M., C.A.O., K.E.K., and E.J.G. held Oak Ridge Institute for Science and Engineering Fellowship awards and E.J.V.-C. held a Geneva fellowship award.
Footnotes
Competing interests: P.A.B. and K.I. are founders and have financial interests in CytoDel Inc. During this study, K.I. consulted for CytoDel Inc., P.A.B. consulted for Palette Life Sciences AB, and C.B.S. consulted for Ology Bioservices Inc. K.I. and P.A.B. are inventors on patent US007785606B2, titled “Genetically engineered clostridial genes, proteins, encoded by the engineered genes, and uses thereof,” and US008980284B2, titled “Recombinant derivatives of botulinum neurotoxins engineered for trafficking studies and neuronal delivery.” K.I., E.J.V.-C., and P.A.B. are inventors on patent application WO2016/094555A1 titled “Clostridial neurotoxin fusion proteins, propeptide fusions, their expression, and use.”
Data and materials availability: All data associated with this study are present in the paper or the Supplementary Materials. Proprietary reagents used during these studies are available upon request from the corresponding author.
SUPPLEMENTARY MATERIALS
REFERENCES AND NOTES
- 1.Pirazzini M, Rossetto O, Eleopra R, Montecucco C, Botulinum neurotoxins: Biology, pharmacology, and toxicology. Pharmacol. Rev 69, 200–235 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnon SS, Schechter R, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Hauer J, Layton M, Lillibridge S, Osterholm MT, O'Toole T, Parker G, Perl TM, Russell PK, Swerdlow DL, Tonat K; Working Group on Civilian Biodefense, Botulinum toxin as a biological weapon: Medical and public health management. JAMA 285, 1059–1070 (2001). [DOI] [PubMed] [Google Scholar]
- 3.Rossetto O, Pirazzini M, Fabris F, Montecucco C, Botulinum neurotoxins: Mechanism of action, in Handbook of Experimental Pharmacology (Springer, 2020). [DOI] [PubMed] [Google Scholar]
- 4.Pirazzini M, Rossetto O, Challenges in searching for therapeutics against botulinum neurotoxins. Expert Opin. Drug Discov 12, 497–510 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Dhaked RK, Singh MK, Singh P, Gupta P, Botulinum toxin: Bioweapon & magic drug. Indian J. Med. Res 132, 489–503 (2010). [PMC free article] [PubMed] [Google Scholar]
- 6.Peck MW, Smith TJ, Anniballi F, Austin JW, Bano L, Bradshaw M, Cuervo P, Cheng LW, Derman Y, Dorner BG, Fisher A, Hill KK, Kalb SR, Korkeala H, Lindstrom M, Lista F, Ldquez C, Mazuet C, Pirazzini M, Popoff MR, Rossetto O, Rummel A, Sesardic D, Singh BR, Stringer SC, Historical perspectives and guidelines for botulinum neurotoxin subtype nomenclature. Toxins 9, 38 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunt J, Carter AT, Stringer SC, Peck MW, Identification of a novel botulinum neurotoxin gene cluster in Enterococcus. FEBS Lett. 592, 310–317 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang S, Masuyer G, Zhang J, Shen Y, Lundin D, Henriksson L, Miyashita SI, Martínez-Carranza M, Dong M, Stenmark P, Identification and characterization of a novel botulinum neurotoxin. Nat. Commun 8, 14130 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hackett G, Moore K, Burgin D, Hornby F, Gray B, Elliott M, Mir I, Beard M, Purification and characterization of recombinant botulinum neurotoxin serotype FA, also known as serotype H. Toxins 10, 195 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossetto O, Pirazzini M, Montecucco C, Botulinum neurotoxins: Genetic, structural and mechanistic insights. Nat. Rev. Microbiol 12, 535–549 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Simpson LL, Identification of the major steps in botulinum toxin action. Annu. Rev. Pharmacol. Toxicol 44, 167–193 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Chaudhry R, Botulism: A diagnostic challenge. Indian J. Med. Res 134, 10–12 (2011). [PMC free article] [PubMed] [Google Scholar]
- 13.Yu PA, Lin NH, Mahon BE, Sobel J, Yu Y, Mody RK, Gu W, Clements J, Kim HJ, Rao AK, Safety and improved clinical outcomes in patients treated with new equine-derived heptavalent botulinum antitoxin. Clin. Infect. Dis 66, S57–S64 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richardson JS, Parrera GS, Astacio H, Sahota H, Anderson DM, Hall C, Babinchak T, Safety and clinical outcomes of an equine-derived heptavalent botulinum antitoxin treatment for confirmed or suspected botulism in the United States. Clin. Infect. Dis 70, 1950–1957 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Larsen JC, Botulinum neurotoxin (BoNT) therapeutics: Time to think outside the BoNT? The Botulinum J. 1, 261–269 (2009). [Google Scholar]
- 16.Smith TJ, Roxas-Duncan V, Smith LA, Botulinum neurotoxins as biothreat agents. J. Bioterr. Biodef s2, 003 (2012). [Google Scholar]
- 17.Vazquez-Cintron EJ, Beske PH, Tenezaca L, Tran BQ, Oyler JM, Glotfelty EJ, Angeles CA, Syngkon A, Mukherjee J, Kalb SR, Band PA, McNutt PM, Shoemaker CB, Ichtchenko K, Engineering botulinum neurotoxin C1 as a molecular vehicle for intra-neuronal drug delivery. Sci. Rep 7, 42923 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tremblay JM, Kuo CL, Abeijon C, Sepulveda J, Oyler G, Hu X, Jin MM, Shoemaker CB, Camelid single domain antibodies (VHHs) as neuronal cell intrabody binding agents and inhibitors of Clostridium botulinum neurotoxin (BoNT) proteases. Toxicon 56, 990–998 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terranova W, Breman JG, Locey RP, Speck S, Botulism type B: Epidemiologic aspects of an extensive outbreak. Am. J. Epidemiol 108, 150–156 (1978). [DOI] [PubMed] [Google Scholar]
- 20.Kodihalli S, Emanuel A, Takla T, Hua Y, Hobbs C, LeClaire R, O’Donnell DC, Therapeutic efficacy of equine botulism antitoxin in rhesus macaques. PLOS ONE 12, e0186892 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukherjee J, Tremblay JM, Leysath CE, Ofori K, Baldwin K, Feng X, Bedenice D, Webb RP, Wright PM, Smith LA, Tzipori S, Shoemaker CB, A novel strategy for development of recombinant antitoxin therapeutics tested in a mouse botulism model. PLOS ONE 7, e29941 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Emanuel A, Qiu H, Barker D, Takla T, Gillum K, Neimuth N, Kodihalli S, Efficacy of equine botulism antitoxin in botulism poisoning in a guinea pig model. PLOS ONE 14, e0209019 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barker D, Gillum KT, Niemuth NA, Kodihalli S, Therapeutic efficacy of equine botulism heptavalent antitoxin against all seven botulinum neurotoxins in symptomatic guinea pigs. PLOS ONE 14, e0222670 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrero BA, Ecklung AE, Streett CS, Ford DF, King JK, Experimental botulism in monkeys—A clinical pathological study. Exp. Mol. Pathol 6, 84–95 (1967). [DOI] [PubMed] [Google Scholar]
- 25.Scott AB, Suzuki D, Systemic toxicity of botulinum toxin by intramuscular injection in the monkey. Mov. Disord 3, 333–335 (1988). [DOI] [PubMed] [Google Scholar]
- 26.Myiashita et al. STM in press.
- 27.Francis JW, Hosler BA, Brown RH Jr., Fishman PS, CuZn superoxide dismutase (SOD-1): Tetanus toxin fragment C hybrid protein for targeted delivery of SOD-1 to neuronal cells. J. Biol. Chem 270, 15434–15442 (1995). [DOI] [PubMed] [Google Scholar]
- 28.Goodnough MC, Oyler G, Fishman PS, Johnson EA, Neale EA, Keller JE, Tepp WH Clark M, Hartz S, Adler M, Development of a delivery vehicle for intracellular transport of botulinum neurotoxin antagonists. FEBS Lett. 513, 163–168 (2002). [DOI] [PubMed] [Google Scholar]
- 29.Zhang P, Ray R, Singh B, Li D, Adler M, Ray P, An efficient drug delivery vehicle for botulism countermeasure. BMC Pharmacol. 9, 12 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh BR, Thirunavukkarasu N, Ghosal K, Ravichandran E, Kukreja R, Cai S, Zhang P, Ray R, Ray P, Clostridial neurotoxins as a drug delivery vehicle targeting nervous system. Biochimie 92, 1252–1259 (2010). [DOI] [PubMed] [Google Scholar]
- 31.Ho M, Chang LH, Pires-Alves M, Thyagarajan B, Bloom JE, Gu Z, Aberle KK, Teymorian SA, Bannai Y, Johnson SC, McArdle JJ, Wilson BA, Recombinant botulinum neurotoxin A heavy chain-based delivery vehicles for neuronal cell targeting. Protein Eng. Des. Sel 24, 247–253 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meunier FA, Lisk G, Sesardic D, Dolly JO, Dynamics of motor nerve terminal remodeling unveiled using SNARE-cleaving botulinum toxins: The extent and duration are dictated by the sites of SNAP-25 truncation. Mol. Cell. Neurosci 22, 454–466 (2003). [DOI] [PubMed] [Google Scholar]
- 33.Rossetto O, Montecucco C, Tables of toxicity of botulinum and tetanus neurotoxins. Toxins 11, 686 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torres VI, Vallejo D, Inestrosa NC, Emerging synaptic molecules as candidates in the etiology of neurological disorders. Neural Plast. 2017, 8081758 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vazquez-Cintron EJ, Vakulenko M, Band PA, Stanker LH, Johnson EA, Ichtchenko K, Atoxic derivative of botulinum neurotoxin A as a prototype molecular vehicle for targeted delivery to the neuronal cytoplasm. PLOS ONE 9, e85517 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lam KH, Tremblay JM, Vazquez-Cintron E, Perry K, Ondeck C, Webb RP, McNutt PM, Shoemaker CB, Jin R, Structural insights into rational design of single-domain antibody-based antitoxins against botulinum neurotoxins. Cell Rep. 30, 2526–2539.e6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt DJ, Beamer G, Tremblay JM, Steele JA, Kim HB, Wang Y, Debatis M, Sun X, Kashentseva EA, Dmitriev IP, Curiel DT, Shoemaker CB, Tzipori S, A tetraspecific VHH-based neutralizing antibody modifies disease outcome in three animal models of clostridium difficile infection. Clin. Vaccine Immunol 23, 774–784 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuo CL, Oyler GA, Shoemaker CB, Accelerated neuronal cell recovery from botulinum neurotoxin intoxication by targeted ubiquitination. PLOS ONE 6, e20352 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caussinus E, Kanca O, Affolter M, Protein knockouts in living eukaryotes using deGradFP and green fluorescent protein fusion targets. Curr. Protoc. Protein Sci 73, 30.2.1–30.2.13 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Nayak SU, Griffiss JM, McKenzie R, Fuchs EJ, Jurao RA, An AT, Ahene A, Tomic M, Hendrix CW, Zenilman JM, Safety and pharmacokinetics of XOMA 3AB, a novel mixture of three monoclonal antibodies against botulinum toxin A. Antimicrob. Agents Chemother 58, 5047–5053 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pirazzini M, Azarnia Tehran D, Zanetti G, Megighian A, Scorzeto M, Fillo S, Shone CC, Binz T, Rossetto O, Lista F, Montecucco C, Thioredoxin and its reductase are present on synaptic vesicles, and their inhibition prevents the paralysis induced by botulinum neurotoxins. Cell Rep. 8, 1870–1878 (2014). [DOI] [PubMed] [Google Scholar]
- 42.Vazquez-Cintron E, Machamer J, Ondeck C, Pagarigan K, Winner B, Bodner P, Kelly K, Pennington MR, McNutt P, Symptomatic treatment of botulism with a clinically approved small molecule. JCI Insight 5, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Touman A, Stratakos, in Tracheal Intubation, Erbay RH, Ed. (IntechOpen, 2018). [Google Scholar]
- 44.Anderson DM, Kumar VR, Arper DL, Kruger E, Bilir SP, Richardson JS, Cost savings associated with timely treatment of botulism with botulism antitoxin heptavalent product. PLOS ONE 14, e0224700 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Foran PG, Mohammed N, Lisk GO, Nagwaney S, Lawrence GW, Johnson E, Smith L, Aoki KR, Dolly JO, Evaluation of the therapeutic usefulness of botulinum neurotoxin B, C1, E, and F compared with the long lasting type A. Basis for distinct durations of inhibition of exocytosis in central neurons. J. Biol. Chem 278, 1363–1371 (2003). [DOI] [PubMed] [Google Scholar]
- 46.Vazquez-Cintron E, Tenezaca L, Angeles C, Syngkon A, Liublinska V, Ichtchenko K, Band P, Pre-clinical study of a novel recombinant botulinum neurotoxin derivative engineered for improved safety. Sci. Rep 6, 30429 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Centers for Disease Control and Prevention (CDC), “Botulism Annual Summary, 2017” (Atlanta, Georgia: U.S. Department of Health and Human Services, CDC, 2019; https://www.cdc.gov/botulism/surv/2017/index.html). [Google Scholar]
- 48.National Research Council (U.S.). Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research (U.S.), National Academies Press (U.S.), Guide for the Care and Use of Laboratory Animals (National Academies Press, 2011), p. xxv, 220 pp. [Google Scholar]
- 49.Pearce LB, Borodic GE, First ER, MacCallum RD, Measurement of botulinum toxin activity: Evaluation of the lethality assay. Toxicol. Appl. Pharmacol 128, 69–77 (1994). [DOI] [PubMed] [Google Scholar]
- 50.Beske PH, Bradford AB, Grynovicki JO, Glotfelty EJ, Hoffman KM, Hubbard KS, Tuznik KM, McNutt PM, Botulinum and tetanus neurotoxin-induced blockade of synaptic transmission in networked cultures of human and rodent neurons. Toxicol. Sci 149, 503–515 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beske PH, Scheeler SM, Adler M, McNutt PM, Accelerated intoxication of GABAergic synapses by botulinum neurotoxin A disinhibits stem cell-derived neuron networks prior to network silencing. Front. Cell. Neurosci 9, 159 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Webb RP, Smith TJ, Wright P, Brown J, Smith LA, Production of catalytically inactive BoNT/A1 holoprotein and comparison with BoNT/A1 subunit vaccines against toxin subtypes A1, A2, and A3. Vaccine 27, 4490–4497 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. sdAb B8 binds LC/A1 but not the intact BoNT/A1 heterodimer.
Fig. S2. B8C1ad expression, purification, and processing.
Fig. S3. Proteomic and immunological characterization of B8C1ad heterodimer.
Fig. S4. Internalized B8LC colocalizes with presynaptic markers in primary neurons.
Fig. S5. Internalized B8LC does not colocalize with endosomal, lysosomal, or autophagosomal markers in primary neurons.
Fig. S6. B8C1ad protects against BoNT/A1 challenge in primary neurons.
Table S1. Dose-dependent toxicities of B8C1ad in mice, guinea pigs, and rhesus macaques.
Table S2. Emergence of respiratory signs of botulism in mice challenged with 2 mipLD50 BoNT/A1.
Table S3. Sample sizes, statistical analyses, and significance thresholds for all comparisons.
Data file S1. Individual-level details for each experiment.
Data file S2. Individual CSS scores at all time points for guinea pig and rhesus macaque survival studies.
Reference (52)